| 1 |
ClinicalTrials.gov (NCT05927649) A TQTc Study for Omaveloxolone
|
| 2 |
FDA Approved Drug Products from FDA Official Website. 2023. Application Number: 216718.
|
| 3 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 4 |
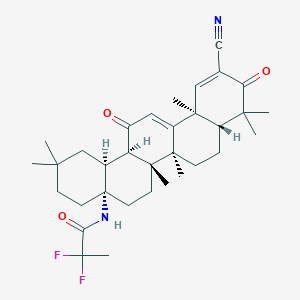
Topical application of the synthetic triterpenoid RTA 408 activates Nrf2 and induces cytoprotective genes in rat skin. Arch Dermatol Res. 2014 Jul;306(5):447-54.
|
| 5 |
Novel agents in the management of Mycobacterium tuberculosis disease. Curr Med Chem. 2007;14(18):2000-8.
|
| 6 |
Moxifloxacin FDA Label
|
| 7 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services.
|
|
|
|
|
|
|