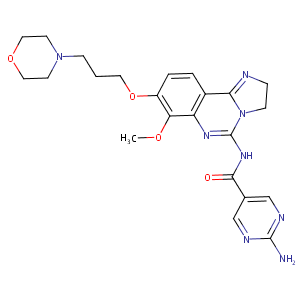
| 1 |
ClinicalTrials.gov (NCT06218667) A Study of Copanlisib in Combination With Degarelix in People With Prostate Cancer
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5585).
|
| 3 |
2017 FDA drug approvals.Nat Rev Drug Discov. 2018 Feb;17(2):81-85.
|
| 4 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 5 |
Iterative approach to the discovery of novel degarelix analogues: substitutions at positions 3, 7, and 8. Part II. J Med Chem. 2005 Jul 28;48(15):4851-60.
|
| 6 |
BAY 80-6946 is a highly selective intravenous PI3K inhibitor with potent p110 and p110 activities in tumor cell lines and xenograft models.Mol Cancer Ther.2013 Nov;12(11):2319-30.
|
| 7 |
FDA label of Copanlisib. The 2020 official website of the U.S. Food and Drug Administration.
|
| 8 |
PI3K/AKT inhibitors aggravate death receptor-mediated hepatocyte apoptosis and liver injury. Toxicol Appl Pharmacol. 2019 Oct 15;381:114729. doi: 10.1016/j.taap.2019.114729. Epub 2019 Aug 22.
|
|
|
|
|
|
|