| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
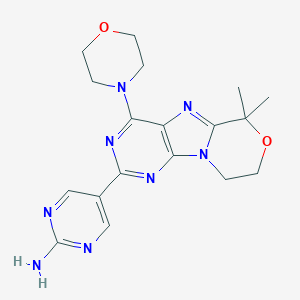
ClinicalTrials.gov (NCT03522298) Safety, Pharmacokinetics and Efficacy of Paxalisib (GDC-0084) in Newly-diagnosed Glioblastoma. U.S. National Institutes of Health.
|
| 3 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7965).
|
| 4 |
Current clinical development of PI3K pathway inhibitors in glioblastoma. Neuro Oncol. 2012 July; 14(7): 819-829.
|
| 5 |
Combination of the PI3K inhibitor ZSTK474 with a PSMA-targeted immunotoxin accelerates apoptosis and regression of prostate cancer. Neoplasia. 2013 Oct;15(10):1172-83.
|
| 6 |
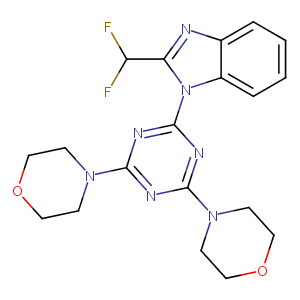
Synthesis and biological evaluation of novel analogues of the pan class I phosphatidylinositol 3-kinase (PI3K) inhibitor 2-(difluoromethyl)-1-[4,6-di(4-morpholinyl)-1,3,5-triazin-2-yl]-1H-benzimidazole (ZSTK474). J Med Chem. 2011 Oct 27;54(20):7105-26. doi: 10.1021/jm200688y. Epub 2011 Sep 27.
|
| 7 |
The dual pathway inhibitor rigosertib is effective in direct patient tumor xenografts of head and neck squamous cell carcinomas. Mol Cancer Ther. 2013 Oct;12(10):1994-2005. doi: 10.1158/1535-7163.MCT-13-0206. Epub 2013 Jul 19.
|
|
|
|
|
|
|