Details of the Drug Combination
General Information of Drug Combination (ID: DC4WE4C)
| Drug Combination Name |
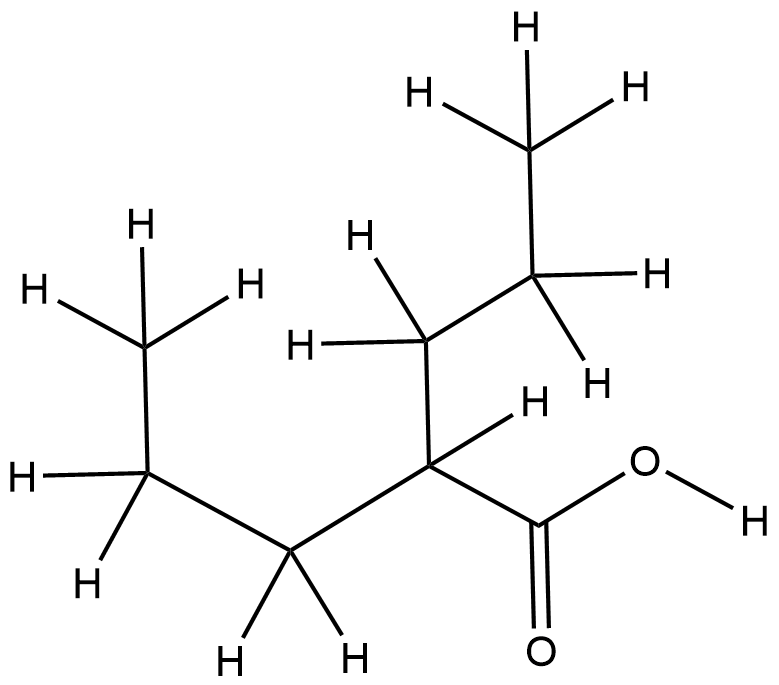
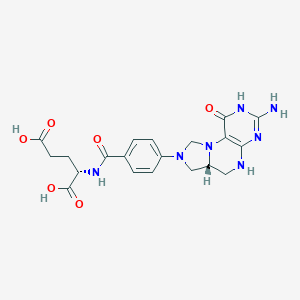
Valproic Acid Arfolitixorin
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indication |
|
|||||||||||||||||
| Component Drugs | Valproic Acid | Arfolitixorin | ||||||||||||||||
| Small molecular drug | Small molecular drug | |||||||||||||||||
|
|
|||||||||||||||||
| 2D MOL | 2D MOL | |||||||||||||||||
| 3D MOL | 3D MOL | |||||||||||||||||
| High-throughput Screening Result | Testing Cell Line: KBM-7 | |||||||||||||||||
| Zero Interaction Potency (ZIP) Score: 9 | ||||||||||||||||||
| Bliss Independence Score: 9 | ||||||||||||||||||
| Loewe Additivity Score: 10.87 | ||||||||||||||||||
| LHighest Single Agent (HSA) Score: 10.87 | ||||||||||||||||||
References
