| 1 |
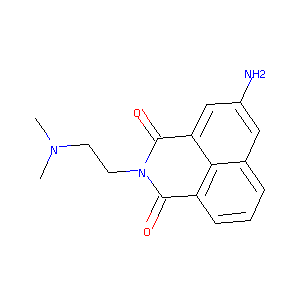
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
ClinicalTrials.gov (NCT00715637) Phase III Randomized Study of Amonafide (AS1413) and Cytarabine Versus Daunorubicin and Cytarabine in Patients With Secondary Acute Myeloid Leukemia (AML)- the ACCEDEStudy. U.S. National Institutes of Health.
|
| 3 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6824).
|
| 4 |
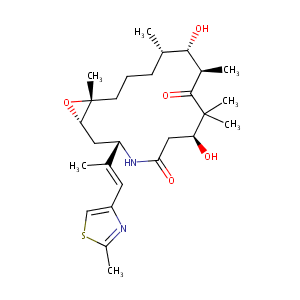
Ixabepilone FDA Label
|
| 5 |
Mullard A: 2010 FDA drug approvals. Nat Rev Drug Discov. 2011 Feb;10(2):82-5.
|
| 6 |
Ixabepilone, a novel microtubule-targeting agent for breast cancer, is a substrate for P-glycoprotein (P-gp/MDR1/ABCB1) but not breast cancer resistance protein (BCRP/ABCG2). J Pharmacol Exp Ther. 2011 May;337(2):423-32.
|
| 7 |
The effect of ketoconazole on the pharmacokinetics and pharmacodynamics of ixabepilone: a first in class epothilone B analogue in late-phase clinical development. Clin Cancer Res. 2008 May 1;14(9):2701-9.
|
|
|
|
|
|
|