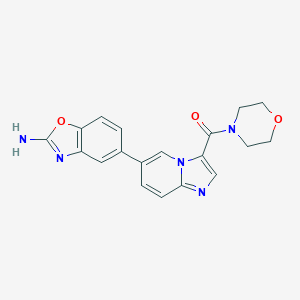
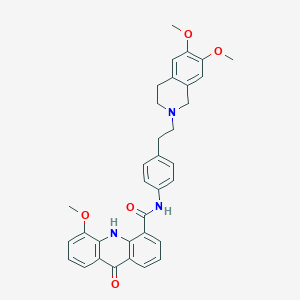
| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
ClinicalTrials.gov (NCT02393209) Docetaxel With or Without MLN1117 in Participants With Locally Advanced or Metastatic Non-small Cell Lung Cancer. U.S. National Institutes of Health.
|
| 3 |
Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800002537)
|
| 4 |
National Cancer Institute Drug Dictionary (drug id 714372).
|
| 5 |
2-[(3-Methoxyphenylethyl)phenoxy]-based ABCB1 inhibitors: effect of different basic side-chains on their biological properties. J Med Chem. 2008 Dec 11;51(23):7602-13.
|
| 6 |
Human organic anion-transporting polypeptide OATP-A (SLC21A3) acts in concert with P-glycoprotein and multidrug resistance protein 2 in the vectorial transport of Saquinavir in Hep G2 cells. Mol Pharm. 2004 Jan 12;1(1):49-56.
|
| 7 |
Flavonoid structure-activity studies identify 6-prenylchrysin and tectochrysin as potent and specific inhibitors of breast cancer resistance protein ABCG2. Cancer Res. 2005 Jun 1;65(11):4852-60.
|
| 8 |
Early identification of clinically relevant drug interactions with the human bile salt export pump (BSEP/ABCB11). Toxicol Sci. 2013 Dec;136(2):328-43.
|
|
|
|
|
|
|