| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
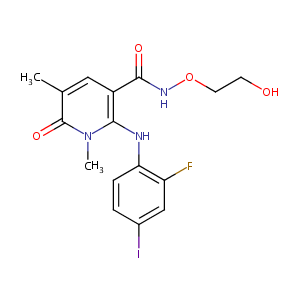
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 8474).
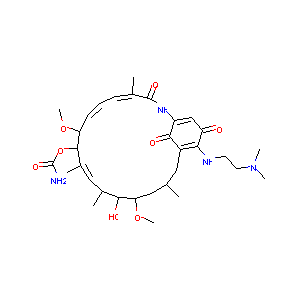
|
| 3 |
ClinicalTrials.gov (NCT00089271) 17-DMAG in Treating Patients With Metastatic or Unresectable Solid Tumors or Lymphomas. U.S. National Institutes of Health.
|
| 4 |
MEK and the inhibitors: from bench to bedside. J Hematol Oncol. 2013; 6: 27.
|
| 5 |
Stage 1 testing and pharmacodynamic evaluation of the HSP90 inhibitor alvespimycin (17-DMAG, KOS-1022) by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008 Jul;51(1):34-41.
|
|
|
|
|
|
|