| 1 |
ClinicalTrials.gov (NCT01828476) Navitoclax and Abiraterone Acetate With or Without Hydroxychloroquine in Treating Patients With Progressive Metastatic Castrate Refractory Prostate Cancer
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6745).
|
| 3 |
ClinicalTrials.gov (NCT04472598) Study of Oral Navitoclax Tablet In Combination With Oral Ruxolitinib Tablet When Compared With Oral Ruxolitinib Tablet To Assess Change In Spleen Volume In Adult Participants With Myelofibrosis (TRANSFORM-1). U.S. National Institutes of Health.
|
| 4 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 8319).
|
| 5 |
Small molecules, big targets: drug discovery faces the protein-protein interaction challenge.Nat Rev Drug Discov. 2016 Aug;15(8):533-50.
|
| 6 |
2011 FDA drug approvals. Nat Rev Drug Discov. 2012 Feb 1;11(2):91-4.
|
| 7 |
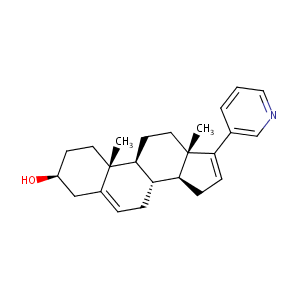
A fission yeast-based test system for the determination of IC50 values of anti-prostate tumor drugs acting on CYP21. J Enzyme Inhib Med Chem. 2006 Oct;21(5):547-56.
|
| 8 |
Elucidating mechanisms of toxicity using phenotypic data from primary human cell systems--a chemical biology approach for thrombosis-related side effects. Int J Mol Sci. 2015 Jan 5;16(1):1008-29. doi: 10.3390/ijms16011008.
|
| 9 |
Targeting CD46 for both adenocarcinoma and neuroendocrine prostate cancer. JCI Insight. 2018 Sep 6;3(17):e121497. doi: 10.1172/jci.insight.121497. eCollection 2018 Sep 6.
|
| 10 |
Targeting chromatin binding regulation of constitutively active AR variants to overcome prostate cancer resistance to endocrine-based therapies. Nucleic Acids Res. 2015 Jul 13;43(12):5880-97. doi: 10.1093/nar/gkv262. Epub 2015 Apr 23.
|
| 11 |
Clinical pipeline report, company report or official report of Roche (2009).
|
| 12 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 13 |
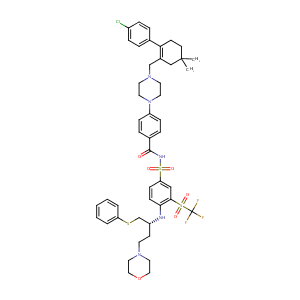
The B-cell lymphoma 2 (BCL2)-inhibitors, ABT-737 and ABT-263, are substrates for P-glycoprotein. Biochem Biophys Res Commun. 2011 May 6;408(2):344-9.
|
| 14 |
Effect of rifampin on the pharmacokinetics, safety and tolerability of navitoclax (ABT-263), a dual inhibitor of Bcl-2 and Bcl-XL , in patients with cancer. J Clin Pharm Ther. 2014 Dec;39(6):680-4.
|
| 15 |
Human breast cancer cells display different sensitivities to ABT-263 based on the level of survivin. Toxicol In Vitro. 2018 Feb;46:229-236. doi: 10.1016/j.tiv.2017.09.023. Epub 2017 Sep 23.
|
| 16 |
BCL2/BCL-X(L) inhibition induces apoptosis, disrupts cellular calcium homeostasis, and prevents platelet activation. Blood. 2011 Jun 30;117(26):7145-54. doi: 10.1182/blood-2011-03-344812. Epub 2011 May 11.
|
|
|
|
|
|
|