| 1 |
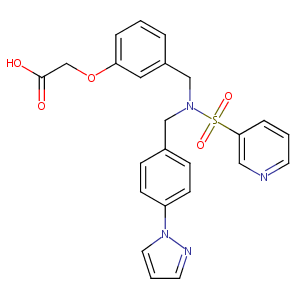
ClinicalTrials.gov (NCT00934089) A Phase 2 Study Of The 24-Hour Intraocular Pressure Lowering And Systemic Exposure Of PF-04217329
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5816).
|
| 3 |
Latanoprost FDA Label
|
| 4 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 1961).
|
| 5 |
Clinical pipeline report, company report or official report of Mati therapeutics.
|
| 6 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 344).
|
| 7 |
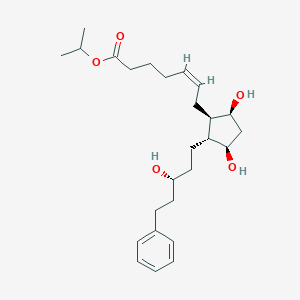
A phase 2, randomized, dose-response trial of taprenepag isopropyl (PF-04217329) versus latanoprost 0.005% in open-angle glaucoma and ocular hypertension. Curr Eye Res. 2011 Sep;36(9):809-17.
|
| 8 |
PGF(2alpha) FP receptor contributes to brain damage following transient focal brain ischemia. Neurotox Res. 2009 Jan;15(1):62-70.
|
| 9 |
The prostaglandin transporter OATP2A1 is expressed in human ocular tissues and transports the antiglaucoma prostanoid latanoprost. Invest Ophthalmol Vis Sci. 2010 May;51(5):2504-11.
|
| 10 |
A multifactorial approach to hepatobiliary transporter assessment enables improved therapeutic compound development. Toxicol Sci. 2013 Nov;136(1):216-41.
|
| 11 |
ClinicalTrials.gov (NCT00572455) Safety and Efficacy of PF-04217329 in Patients With Glaucoma or Elevated Eye Pressure.
|
|
|
|
|
|
|