| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
ClinicalTrials.gov (NCT03750786) A Study to Compare the Efficacy of Arfolitixorin Versus Leucovorin in Combination With 5 Fluorouracil, Oxaliplatin, and Bevacizumab in Patients With Advanced Colorectal Cancer (AGENT). U.S. National Institutes of Health.
|
| 3 |
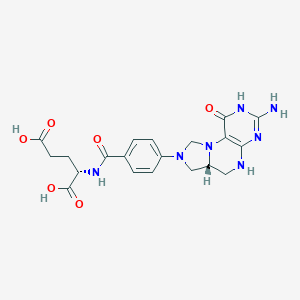
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7486).
|
| 4 |
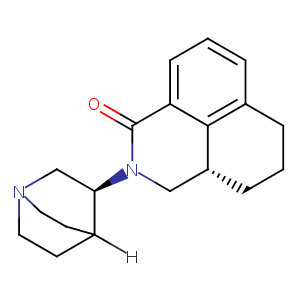
Palonosetron FDA Label
|
| 5 |
Management of postoperative nausea and vomiting: focus on palonosetron. Ther Clin Risk Manag. 2009 Feb;5(1):21-34.
|
| 6 |
Pharmacokinetics, metabolism and excretion of intravenous [l4C]-palonosetron in healthy human volunteers. Biopharm Drug Dispos. 2004 Nov;25(8):329-37.
|
| 7 |
Cytochrome P450 2D6 metabolism and 5-hydroxytryptamine type 3 receptor antagonists for postoperative nausea and vomiting. Med Sci Monit. 2005 Oct;11(10):RA322-8.
|
| 8 |
Why are most phospholipidosis inducers also hERG blockers?. Arch Toxicol. 2017 Dec;91(12):3885-3895. doi: 10.1007/s00204-017-1995-9. Epub 2017 May 27.
|
|
|
|
|
|
|