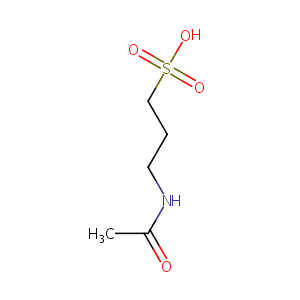
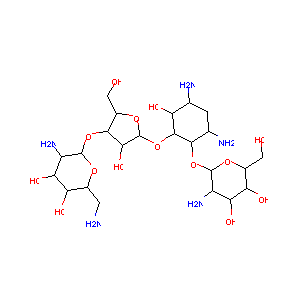
| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7106).
|
| 3 |
Paromomycin FDA Label
|
| 4 |
FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 064171.
|
| 5 |
Predicting the effect of naltrexone and acamprosate in alcohol-dependent patients using genetic indicators. Addict Biol. 2009 Jul;14(3):328-37.
|
| 6 |
Aminoglycoside association pathways with the 30S ribosomal subunit. J Phys Chem B. 2009 May 21;113(20):7322-30.
|
| 7 |
Correction of ATM gene function by aminoglycoside-induced read-through of premature termination codons. Proc Natl Acad Sci U S A. 2004 Nov 2;101(44):15676-81. doi: 10.1073/pnas.0405155101. Epub 2004 Oct 21.
|
|
|
|
|
|
|