| 1 |
ClinicalTrials.gov (NCT02882477) Treatment of Wolfram Syndrome Type 2 With the Chelator Deferiprone and Incretin Based Therapy
|
| 2 |
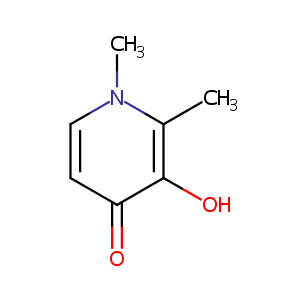
Deferiprone FDA Label
|
| 3 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7456).
|
| 4 |
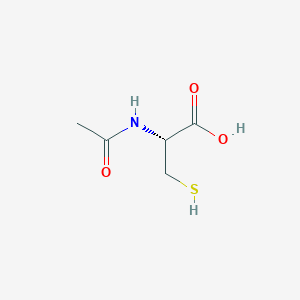
Acetylcysteine FDA Label
|
| 5 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 6 |
2011 FDA drug approvals. Nat Rev Drug Discov. 2012 Feb 1;11(2):91-4.
|
| 7 |
Deferiprone glucuronidation by human tissues and recombinant UDP glucuronosyltransferase 1A6: an in vitro investigation of genetic and splice variants. Drug Metab Dispos. 2009 Feb;37(2):322-9.
|
| 8 |
Pharmacological screening using an FXN-EGFP cellular genomic reporter assay for the therapy of Friedreich ataxia. PLoS One. 2013;8(2):e55940.
|
| 9 |
Antiproliferative effect of deferiprone on the Hep G2 cell line. Biochem Pharmacol. 1998 Aug 15;56(4):431-7. doi: 10.1016/s0006-2952(98)00071-9.
|
|
|
|
|
|
|