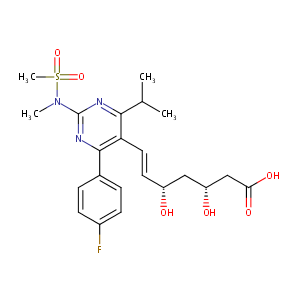
| 1 |
ClinicalTrials.gov (NCT03801733) Drug-Drug Interaction Study of Vadadustat With Rosuvastatin, Sulfasalazine, Pravastatin, Atorvastatin and Simvastatin
|
| 2 |
ClinicalTrials.gov (NCT03242967) Study to Evaluate Three Times Per Week (TIW) Oral Dosing of Vadadustat for Anemia in Subjects With Dialysis-Dependent Chronic Kidney Disease (DD-CKD) (TRILOGY). U.S. National Institutes of Health.
|
| 3 |
Rosuvastatin FDA Label
|
| 4 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2954).
|
| 5 |
DOI: 10.1038/nrd4422
|
| 6 |
New dimension of statin action on ApoB atherogenicity. Clin Cardiol. 2003 Jan;26(1 Suppl 1):I7-10.
|
| 7 |
Involvement of multiple transporters in the hepatobiliary transport of rosuvastatin. Drug Metab Dispos. 2008 Oct;36(10):2014-23.
|
| 8 |
Drug and bile acid transporters in rosuvastatin hepatic uptake: function, expression, and pharmacogenetics. Gastroenterology. 2006 May;130(6):1793-806.
|
| 9 |
The contribution of organic anion transporters OAT1 and OAT3 to the renal uptake of rosuvastatin. J Pharmacol Exp Ther. 2007 Sep;322(3):1221-7.
|
| 10 |
Effects of acid and lactone forms of eight HMG-CoA reductase inhibitors on CYP-mediated metabolism and MDR1-mediated transport. Pharm Res. 2006 Mar;23(3):506-12.
|
| 11 |
Pharmacokinetics of rosuvastatin when coadministered with rifampicin in healthy males: a randomized, single-blind, placebo-controlled, crossover study. Clin Ther. 2008 Jul;30(7):1283-9.
|
| 12 |
ABCG2 polymorphism is associated with the low-density lipoprotein cholesterol response to rosuvastatin. Clin Pharmacol Ther. 2010 May;87(5):558-62.
|
| 13 |
Association of the Trp719Arg polymorphism in kinesin-like protein 6 with myocardial infarction and coronary heart disease in 2 prospective trials: the CARE and WOSCOPS trials. J Am Coll Cardiol. 2008 Jan 29;51(4):435-43. doi: 10.1016/j.jacc.2007.05.057.
|
| 14 |
Pharmacogenetic study of statin therapy and cholesterol reduction. JAMA. 2004 Jun 16;291(23):2821-7. doi: 10.1001/jama.291.23.2821.
|
|
|
|
|
|
|