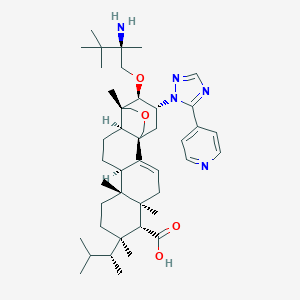
| 1 |
ClinicalTrials.gov (NCT05178862) A Phase 3, Randomized, Double-blind Study for Patients With Invasive Candidiasis Treated With IV Echinocandin Followed by Either Oral Ibrexafungerp or Oral Fluconazole
|
| 2 |
Caspofungin FDA Label
|
| 3 |
Echinocandins: pharmacokinetic and therapeutic issues. Curr Med Res Opin. 2009 Jul;25(7):1741-50.
|
| 4 |
FDA Approved Drug Products from FDA Official Website. 2022. Application Number: 214900.
|
| 5 |
ClinicalTrials.gov (NCT03059992) Study to Evaluate the Efficacy and Safety of Ibrexafungerp in Patients With Fungal Diseases That Are Refractory to or Intolerant of Standard Antifungal Treatment (FURI). U.S. National Institutes of Health.
|
| 6 |
Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800031918)
|
| 7 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services.
|
| 8 |
Organic anion transporting polypeptide 1B1: a genetically polymorphic transporter of major importance for hepatic drug uptake. Pharmacol Rev. 2011 Mar;63(1):157-81.
|
| 9 |
Pharmacodynamic target evaluation of a novel oral glucan synthase inhibitor, SCY-078 (MK-3118), using an in vivo murine invasive candidiasis model. Antimicrob Agents Chemother. 2015 Feb;59(2):1265-72.
|
|
|
|
|
|
|