Details of the Drug Combination
General Information of Drug Combination (ID: DCFVBUO)
| Drug Combination Name |
Amonafide Terameprocol
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indication |
|
|||||||||||||||||
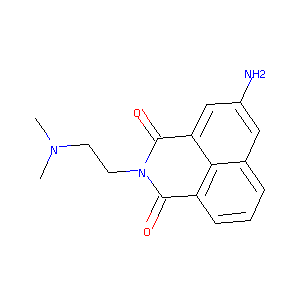
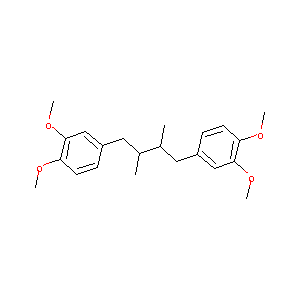
| Component Drugs | Amonafide | Terameprocol | ||||||||||||||||
| Small molecular drug | Small molecular drug | |||||||||||||||||
|
|
|||||||||||||||||
| 2D MOL | 2D MOL | |||||||||||||||||
| 3D MOL | 3D MOL | |||||||||||||||||
| High-throughput Screening Result | Testing Cell Line: MDA-MB-468 | |||||||||||||||||
| Zero Interaction Potency (ZIP) Score: 1.12 | ||||||||||||||||||
| Bliss Independence Score: 1.74 | ||||||||||||||||||
| Loewe Additivity Score: 0.17 | ||||||||||||||||||
| LHighest Single Agent (HSA) Score: 2.81 | ||||||||||||||||||
Molecular Interaction Atlas of This Drug Combination
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indication(s) of Amonafide |
|
|||||||||||||||||||||||||||
| Indication(s) of Terameprocol |
|
|||||||||||||||||||||||||||
|
Terameprocol Interacts with 1 DTT Molecule(s)
|
||||||||||||||||||||||||||||
References
