| 1 |
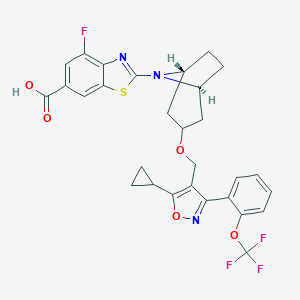
ClinicalTrials.gov (NCT03517540) Study of Safety, Tolerability, and Efficacy of a Combination Treatment of LJN452 and CVC in Adult Patients With NASH and Liver Fibrosis
|
| 2 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 3 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 801).
|
| 4 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 5 |
FXR modulators for enterohepatic and metabolic diseases.Expert Opin Ther Pat. 2018 Nov;28(11):765-782.
|
| 6 |
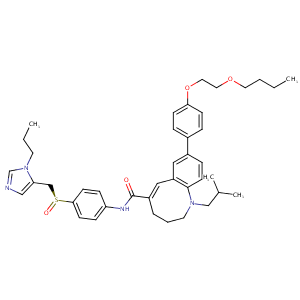
The dual CCR5 and CCR2 inhibitor cenicriviroc does not redistribute HIV into extracellular space: implications for plasma viral load and intracellular DNA decline. J Antimicrob Chemother. 2015 Mar;70(3):750-6.
|
| 7 |
Pharmacokinetics, safety, and CCR2/CCR5 antagonist activity of cenicriviroc in participants with mild or moderate hepatic impairment. Clin Transl Sci. 2016 Jun;9(3):139-48.
|
|
|
|
|
|
|