| 1 |
Loss of function mutations in VARS encoding cytoplasmic valyl-tRNA synthetase cause microcephaly, seizures, and progressive cerebral atrophy.Hum Genet. 2018 Apr;137(4):293-303. doi: 10.1007/s00439-018-1882-3. Epub 2018 Apr 24.
|
| 2 |
FDA Approved Drug Products from FDA Official Website. 2021. Application Number: 214622.
|
| 3 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7877).
|
| 4 |
Discovery of a novel ERK inhibitor with activity in models of acquired resistance to BRAF and MEK inhibitors. Cancer Discov. 2013 Jul;3(7):742-50.
|
| 5 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 6 |
Pharmacological inhibition of fibroblast growth factor (FGF) receptor signaling ameliorates FGF23-mediated hypophosphatemic rickets. J Bone Miner Res. 2013 Apr;28(4):899-911.
|
| 7 |
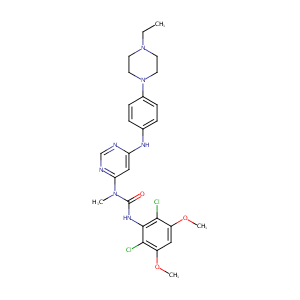
Discovery of 3-(2,6-dichloro-3,5-dimethoxy-phenyl)-1-{6-[4-(4-ethyl-piperazin-1-yl)-phenylamino]-pyrimidin-4-yl}-1-methyl-urea (NVP-BGJ398), a potent and selective inhibitor of the fibroblast growth factor receptor family of receptor tyrosine kinase. J Med Chem. 2011 Oct 27;54(20):7066-83. doi: 10.1021/jm2006222. Epub 2011 Sep 21.
|
| 8 |
3,4,5-Tri-O-caffeoylquinic acid methyl ester isolated from Lonicera japonica Thunb. Flower buds facilitates hepatitis B virus replication in HepG2.2.15 cells. Food Chem Toxicol. 2020 Apr;138:111250. doi: 10.1016/j.fct.2020.111250. Epub 2020 Mar 7.
|
|
|
|
|
|
|