| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
FDA Approved Drug Products from FDA Official Website. 2019. Application Number: (NDA) 205832
|
| 3 |
Nintedanib FDA Label
|
| 4 |
ClinicalTrials.gov (NCT04338802) Efficacy and Safety of Nintedanib in the Treatment of Pulmonary Fibrosis in Patients With Moderate to Severe COVID -19. U.S. National Institutes of Health.
|
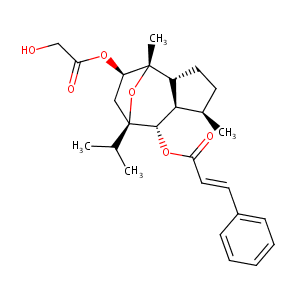
| 5 |
Enantioselective synthesis of (-)-englerins A and B. Angew Chem Int Ed Engl. 2010 May 3;49(20):3517-9.
|
| 6 |
(-)-Englerin is a potent and selective activator of TRPC4 and TRPC5 calcium channels. Angew Chem Int Ed Engl. 2015 Mar 16;54(12):3787-91.
|
|
|
|
|
|
|