| 1 |
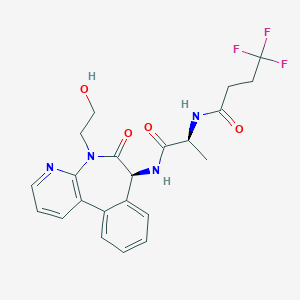
ClinicalTrials.gov (NCT02784795) A Study of LY3039478 in Participants With Advanced or Metastatic Solid Tumors. U.S. National Institutes of Health.
|
| 2 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 3 |
ClinicalTrials.gov (NCT01695005) A Study of LY3039478 in Participants With Advanced Cancer. U.S. National Institutes of Health.
|
| 4 |
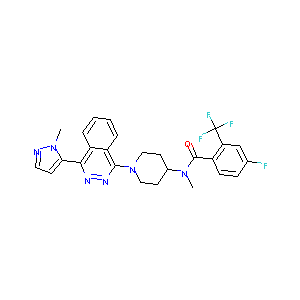
ClinicalTrials.gov (NCT01722292) A Study of LY2940680 in Small Cell Lung Cancer. U.S. National Institutes of Health.
|
| 5 |
J Clin Oncol 33, 2015 (suppl; abstr 2533).
|
| 6 |
Interpreting expression profiles of cancers by genome-wide survey of breadth of expression in normal tissues. Genomics 2005 Aug;86(2):127-41.
|
| 7 |
Phase I study of LY2940680, a Smo antagonist, in patients with advanced cancer including treatment-naive and previously treated basal cell carcinoma. Clin Cancer Res. 2018 May 1;24(9):2082-2091.
|
|
|
|
|
|
|