| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
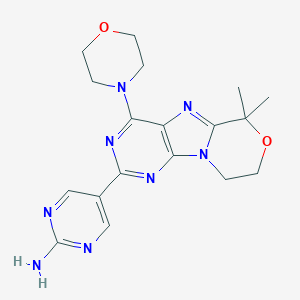
ClinicalTrials.gov (NCT03522298) Safety, Pharmacokinetics and Efficacy of Paxalisib (GDC-0084) in Newly-diagnosed Glioblastoma. U.S. National Institutes of Health.
|
| 3 |
Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800006527)
|
| 4 |
Current clinical development of PI3K pathway inhibitors in glioblastoma. Neuro Oncol. 2012 July; 14(7): 819-829.
|
|
|
|
|
|
|