| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
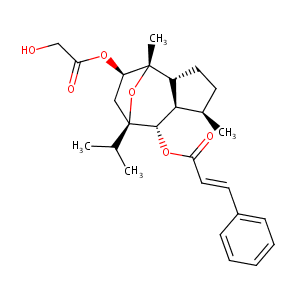
Enantioselective synthesis of (-)-englerins A and B. Angew Chem Int Ed Engl. 2010 May 3;49(20):3517-9.
|
| 3 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5433).
|
| 4 |
(-)-Englerin is a potent and selective activator of TRPC4 and TRPC5 calcium channels. Angew Chem Int Ed Engl. 2015 Mar 16;54(12):3787-91.
|
| 5 |
Estrogen regulation in human breast cancer cells of new downstream gene targets involved in estrogen metabolism, cell proliferation and cell transformation. J Mol Endocrinol. 2004 Apr;32(2):397-414.
|
| 6 |
Estrogen regulation of the glucuronidation enzyme UGT2B15 in estrogen receptor-positive breast cancer cells. Endocrinology. 2006 Aug;147(8):3843-50.
|
| 7 |
Inhibition of thymidine phosphorylase expression by Hsp90 inhibitor potentiates the cytotoxic effect of salinomycin in human non-small-cell lung cancer cells. Toxicology. 2019 Apr 1;417:54-63.
|
| 8 |
Suppressed translation and ULK1 degradation as potential mechanisms of autophagy limitation under prolonged starvation. Autophagy. 2016 Nov;12(11):2085-2097. doi: 10.1080/15548627.2016.1226733. Epub 2016 Sep 14.
|
| 9 |
Cinobufagin restrains the growth and triggers DNA damage of human hepatocellular carcinoma cells via proteasome-dependent degradation of thymidylate synthase. Chem Biol Interact. 2022 Jun 1;360:109938. doi: 10.1016/j.cbi.2022.109938. Epub 2022 Apr 12.
|
| 10 |
Induction of vascular endothelial growth factor expression and hypoxia-inducible factor 1alpha protein by the oxidative stressor arsenite. J Biol Chem. 2001 Dec 21;276(51):48066-76. doi: 10.1074/jbc.M106282200. Epub 2001 Oct 18.
|
| 11 |
Population-based in vitro hazard and concentration-response assessment of chemicals: the 1000 genomes high-throughput screening study. Environ Health Perspect. 2015 May;123(5):458-66. doi: 10.1289/ehp.1408775. Epub 2015 Jan 13.
|
| 12 |
Carotenoids reverse multidrug resistance in cancer cells by interfering with ABC-transporters. Phytomedicine. 2012 Aug 15;19(11):977-87.
|
| 13 |
A structure-function relationship among reserpine and yohimbine analogues in their ability to increase expression of mdr1 and P-glycoprotein in a human colon carcinoma cell line. Mol Pharmacol. 1995 Oct;48(4):682-9.
|
| 14 |
Superinduction of CYP1A1 in MCF10A cultures by cycloheximide, anisomycin, and puromycin: a process independent of effects on protein translation and unrelated to suppression of aryl hydrocarbon receptor proteolysis by the proteasome. Mol Pharmacol. 2004 Oct;66(4):936-47.
|
| 15 |
Novel sulfonanilide analogues suppress aromatase expression and activity in breast cancer cells independent of COX-2 inhibition. J Med Chem. 2006 Feb 23;49(4):1413-9.
|
| 16 |
Comparative analysis of AhR-mediated TCDD-elicited gene expression in human liver adult stem cells. Toxicol Sci. 2009 Nov;112(1):229-44.
|
| 17 |
The effect of interferon-alpha on the expression of cytochrome P450 3A4 in human hepatoma cells. Toxicol Appl Pharmacol. 2011 Jun 1;253(2):130-6.
|
| 18 |
Metabolism of bilirubin by human cytochrome P450 2A6. Toxicol Appl Pharmacol. 2012 May 15;261(1):50-8.
|
| 19 |
Aryl hydrocarbon receptor-dependent up-regulation of the heterodimeric amino acid transporter LAT1 (SLC7A5)/CD98hc (SLC3A2) by diesel exhaust particle extract in human bronchial epithelial cells. Toxicol Appl Pharmacol. 2016 Jan 1;290:74-85.
|
| 20 |
Instability of the human cytochrome p450 reductase A287P variant is the major contributor to its antley-bixler syndrome-like phenotype. J Biol Chem. 2016 Sep 23;291(39):20487-502.
|
| 21 |
Possible roles of a tumor suppressor gene PIG11 in hepatocarcinogenesis and As2O3-induced apoptosis in liver cancer cells. J Gastroenterol. 2009;44(5):460-9. doi: 10.1007/s00535-009-0030-1. Epub 2009 Apr 1.
|
| 22 |
Death receptor regulation and celecoxib-induced apoptosis in human lung cancer cells. J Natl Cancer Inst. 2004 Dec 1;96(23):1769-80. doi: 10.1093/jnci/djh322.
|
| 23 |
Tetrachlorobenzoquinone induces Nrf2 activation via rapid Bach1 nuclear export/ubiquitination and JNK-P62 signaling. Toxicology. 2016 Jul 1;363-364:48-57. doi: 10.1016/j.tox.2016.07.002. Epub 2016 Jul 5.
|
| 24 |
The DNA damaging agent VP16 induces the expression of a subset of ligands from the EGF system in bladder cancer cells, whereas none of the four EGF receptors are induced. Mol Cell Biochem. 2004 May;260(1-2):129-35. doi: 10.1023/b:mcbi.0000026063.96267.98.
|
| 25 |
Inhibition of phosphatidylinositol 3-kinase sensitizes vascular endothelial cells to cytokine-initiated cathepsin-dependent apoptosis. J Biol Chem. 2003 Jun 6;278(23):21295-306. doi: 10.1074/jbc.M212837200. Epub 2003 Mar 27.
|
| 26 |
Chronic oxidative stress promotes GADD34-mediated phosphorylation of the TAR DNA-binding protein TDP-43, a modification linked to neurodegeneration. J Biol Chem. 2018 Jan 5;293(1):163-176. doi: 10.1074/jbc.M117.814111. Epub 2017 Nov 6.
|
| 27 |
Transcriptional regulation of UCP4 by NF-kappaB and its role in mediating protection against MPP+ toxicity. Free Radic Biol Med. 2010 Jul 15;49(2):192-204. doi: 10.1016/j.freeradbiomed.2010.04.002. Epub 2010 Apr 9.
|
| 28 |
Induction of c-fos proto-oncogene in mesangial cells by cadmium. J Biol Chem. 1998 Jan 2;273(1):73-9. doi: 10.1074/jbc.273.1.73.
|
| 29 |
Quercetin up-regulates LDL receptor expression in HepG2 cells. Phytother Res. 2012 Nov;26(11):1688-94. doi: 10.1002/ptr.4646. Epub 2012 Mar 3.
|
| 30 |
Identification and Profiling of Environmental Chemicals That Inhibit the TGF/SMAD Signaling Pathway. Chem Res Toxicol. 2019 Dec 16;32(12):2433-2444. doi: 10.1021/acs.chemrestox.9b00228. Epub 2019 Nov 11.
|
| 31 |
Xenoestrogens alter estrogen receptor (ER) intracellular levels. PLoS One. 2014 Feb 20;9(2):e88961. doi: 10.1371/journal.pone.0088961. eCollection 2014.
|
| 32 |
IkappaBalpha (inhibitory kappaBalpha) identified as labile repressor of MnSOD (manganese superoxide dismutase) expression. Biochem J. 2004 Dec 15;384(Pt 3):543-9. doi: 10.1042/BJ20040714.
|
| 33 |
Characterization of cytochrome P4502E1 turnover in transfected HepG2 cells expressing human CYP2E1. Arch Biochem Biophys. 1997 May 1;341(1):25-33. doi: 10.1006/abbi.1997.9907.
|
| 34 |
The synthetic bile acid-phospholipid conjugate ursodeoxycholyl lysophosphatidylethanolamide suppresses TNF-induced liver injury. J Hepatol. 2011 Apr;54(4):674-84. doi: 10.1016/j.jhep.2010.07.028. Epub 2010 Sep 27.
|
| 35 |
Effect of cytokines on ICAM-1 and ZO-1 expression on human airway epithelial cells. Cell Biol Int. 2005 Sep;29(9):768-77. doi: 10.1016/j.cellbi.2005.05.002.
|
| 36 |
Linc-ROR drive adriamycin resistance by targeting AP-2/Wnt/-catenin axis in hepatocellular carcinoma. Cell Biol Toxicol. 2023 Aug;39(4):1735-1752. doi: 10.1007/s10565-022-09777-3. Epub 2022 Dec 28.
|
| 37 |
Dual regulation of 2-adrenoceptor messenger RNA expression in human lung fibroblasts by 2-cAMP signaling; delayed upregulated inhibitors oppose a rapid in onset, direct stimulation of gene expression. Naunyn Schmiedebergs Arch Pharmacol. 2014 Jul;387(7):649-57. doi: 10.1007/s00210-014-0971-7. Epub 2014 Apr 8.
|
| 38 |
RNF168 suppresses the cancer stem cell-like traits of nonsmall cell lung cancer cells by mediating RhoC ubiquitination. Environ Toxicol. 2022 Mar;37(3):603-611. doi: 10.1002/tox.23428. Epub 2021 Dec 7.
|
| 39 |
Inhibition of breast cancer cell growth and induction of cell death by 1,1-bis(3'-indolyl)methane (DIM) and 5,5'-dibromoDIM. Cancer Lett. 2006 May 18;236(2):198-212. doi: 10.1016/j.canlet.2005.05.036. Epub 2005 Jul 26.
|
| 40 |
Deoxynivalenol affects in vitro intestinal epithelial cell barrier integrity through inhibition of protein synthesis. Toxicol Appl Pharmacol. 2010 Jun 15;245(3):291-8. doi: 10.1016/j.taap.2010.03.012. Epub 2010 Apr 1.
|
| 41 |
Redox-sensitive regulation of gene expression in human primary macrophages exposed to inorganic arsenic. J Cell Biochem. 2009 Jun 1;107(3):537-47. doi: 10.1002/jcb.22155.
|
| 42 |
ERR contributes to HDAC6-induced chemoresistance of osteosarcoma cells. Cell Biol Toxicol. 2023 Jun;39(3):813-825. doi: 10.1007/s10565-021-09651-8. Epub 2021 Sep 15.
|
| 43 |
Receptors for bitter, sweet and umami taste couple to inhibitory G protein signaling pathways. Eur J Pharmacol. 2004 Apr 12;489(3):139-49. doi: 10.1016/j.ejphar.2004.03.004.
|
| 44 |
Proliferative suppression by CDK4/6 inhibition: complex function of the retinoblastoma pathway in liver tissue and hepatoma cells. Gastroenterology. 2010 May;138(5):1920-30. doi: 10.1053/j.gastro.2010.01.007. Epub 2010 Jan 25.
|
| 45 |
Solubility shift and SUMOylaltion of promyelocytic leukemia (PML) protein in response to arsenic(III) and fate of the SUMOylated PML. Toxicol Appl Pharmacol. 2015 Sep 15;287(3):191-201. doi: 10.1016/j.taap.2015.05.018. Epub 2015 Jun 3.
|
| 46 |
Akt activation by Ca(2+)/calmodulin-dependent protein kinase kinase 2 (CaMKK2) in ovarian cancer cells. J Biol Chem. 2017 Aug 25;292(34):14188-14204. doi: 10.1074/jbc.M117.778464. Epub 2017 Jun 20.
|
| 47 |
Induction of adrenomedullin during hypoxia in cultured human glioblastoma cells. J Neurochem. 2000 Nov;75(5):1826-33. doi: 10.1046/j.1471-4159.2000.0751826.x.
|
| 48 |
Arsenic trioxide induces autophagic degradation of the FLT3-ITD mutated protein in FLT3-ITD acute myeloid leukemia cells. J Cancer. 2020 Mar 13;11(12):3476-3482. doi: 10.7150/jca.29751. eCollection 2020.
|
| 49 |
Utilization of CDKN1A/p21 gene for class discrimination of DNA damage-induced clastogenicity. Toxicology. 2014 Jan 6;315:8-16. doi: 10.1016/j.tox.2013.10.009. Epub 2013 Nov 6.
|
| 50 |
G1P3, an interferon inducible gene 6-16, is expressed in gastric cancers and inhibits mitochondrial-mediated apoptosis in gastric cancer cell line TMK-1 cell. Cancer Immunol Immunother. 2005 Aug;54(8):729-40. doi: 10.1007/s00262-004-0645-2. Epub 2005 Feb 1.
|
| 51 |
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) exposure of normal human dermal fibroblasts results in AhR-dependent and -independent changes in gene expression. Toxicol Appl Pharmacol. 2007 Apr 1;220(1):9-17. doi: 10.1016/j.taap.2006.12.002. Epub 2006 Dec 15.
|
| 52 |
Arsenic-induced SUMO-dependent recruitment of RNF4 into PML nuclear bodies. Mol Biol Cell. 2010 Dec;21(23):4227-39. doi: 10.1091/mbc.E10-05-0449. Epub 2010 Oct 13.
|
| 53 |
Antiviral Potential of Small Molecules Cordycepin, Thymoquinone, and N6, N6-Dimethyladenosine Targeting SARS-CoV-2 Entry Protein ADAM17. Molecules. 2022 Dec 19;27(24):9044. doi: 10.3390/molecules27249044.
|
| 54 |
A Gene Expression Biomarker Predicts Heat Shock Factor 1 Activation in a Gene Expression Compendium. Chem Res Toxicol. 2021 Jul 19;34(7):1721-1737. doi: 10.1021/acs.chemrestox.0c00510. Epub 2021 Jun 25.
|
| 55 |
Interferon-alpha (Intron A) upregulates urokinase-type plasminogen activator receptor gene expression. Cancer Immunol Immunother. 2002 Jul;51(5):248-54. doi: 10.1007/s00262-002-0275-5. Epub 2002 Apr 9.
|
| 56 |
Inactivation of IkappaB contributes to transcriptional activation of spermidine/spermine N(1)-acetyltransferase. Mol Carcinog. 2006 Sep;45(9):685-93. doi: 10.1002/mc.20239.
|
| 57 |
Presenilin-2 regulates the degradation of RBP-Jk protein through p38 mitogen-activated protein kinase. J Cell Sci. 2012 Mar 1;125(Pt 5):1296-308. doi: 10.1242/jcs.095984. Epub 2012 Feb 2.
|
| 58 |
Dexamethasone suppresses apoptosis in a human gastric cancer cell line through modulation of bcl-x gene expression. FEBS Lett. 1997 Sep 22;415(1):11-5. doi: 10.1016/s0014-5793(97)01083-1.
|
| 59 |
Interleukin-15 delays human neutrophil apoptosis by intracellular events and not via extracellular factors: role of Mcl-1 and decreased activity of caspase-3 and caspase-8. J Leukoc Biol. 2004 May;75(5):893-900. doi: 10.1189/jlb.1103585. Epub 2004 Feb 24.
|
| 60 |
Disease-associated mutations of TDP-43 promote turnover of the protein through the proteasomal pathway. Mol Neurobiol. 2014 Dec;50(3):1049-58. doi: 10.1007/s12035-014-8644-6. Epub 2014 Jan 30.
|
| 61 |
Enhanced p62-NRF2 Feedback Loop due to Impaired Autophagic Flux Contributes to Arsenic-Induced Malignant Transformation of Human Keratinocytes. Oxid Med Cell Longev. 2019 Oct 30;2019:1038932. doi: 10.1155/2019/1038932. eCollection 2019.
|
| 62 |
Resistance to Fas-mediated apoptosis is restored by cycloheximide through the downregulation of cellular FLIPL in NK/T-cell lymphoma. Lab Invest. 2005 Jul;85(7):874-84. doi: 10.1038/labinvest.3700291.
|
| 63 |
Nrf2 Expression and Apoptosis in Quercetin-treated Malignant Mesothelioma Cells. Mol Cells. 2015 May;38(5):416-25. doi: 10.14348/molcells.2015.2268. Epub 2015 Apr 21.
|
| 64 |
NDRG1 inhibition sensitizes osteosarcoma cells to combretastatin A-4 through targeting autophagy. Cell Death Dis. 2017 Sep 14;8(9):e3048. doi: 10.1038/cddis.2017.438.
|
| 65 |
The human type 2 iodothyronine deiodinase is a selenoprotein highly expressed in a mesothelioma cell line. J Biol Chem. 2001 Aug 10;276(32):30183-7. doi: 10.1074/jbc.C100325200. Epub 2001 Jun 25.
|
| 66 |
Protein synthesis inhibitors, in synergy with 5-azacytidine, restore sodium/iodide symporter gene expression in human thyroid adenoma cell line, KAK-1, suggesting trans-active transcriptional repressor. J Clin Endocrinol Metab. 2007 Mar;92(3):1080-7. doi: 10.1210/jc.2006-2106. Epub 2006 Dec 12.
|
| 67 |
MTERF3 contributes to MPP+-induced mitochondrial dysfunction in SH-SY5Y cells. Acta Biochim Biophys Sin (Shanghai). 2022 Aug 25;54(8):1113-1121. doi: 10.3724/abbs.2022098.
|
| 68 |
JNK activation-mediated nuclear SIRT1 protein suppression contributes to silica nanoparticle-induced pulmonary damage via p53 acetylation and cytoplasmic localisation. Toxicology. 2019 Jul 1;423:42-53. doi: 10.1016/j.tox.2019.05.003. Epub 2019 May 11.
|
| 69 |
A MALAT1/HIF-2 feedback loop contributes to arsenite carcinogenesis. Oncotarget. 2016 Feb 2;7(5):5769-87. doi: 10.18632/oncotarget.6806.
|
| 70 |
The RNA polymerase III repressor MAF1 is regulated by ubiquitin-dependent proteasome degradation and modulates cancer drug resistance and apoptosis. J Biol Chem. 2019 Dec 13;294(50):19255-19268. doi: 10.1074/jbc.RA119.008849. Epub 2019 Oct 23.
|
| 71 |
Position of STAT-1 alpha in cycloheximide-dependent apoptosis triggered by TNF-alpha in human colorectal COLO 205 cancer cell line; role of polyphenolic compounds. J Physiol Pharmacol. 2005 Jun;56 Suppl 3:119-41.
|
| 72 |
Reduced level of ribonucleotide reductase R2 subunits increases dependence on homologous recombination repair of cisplatin-induced DNA damage. Mol Pharmacol. 2011 Dec;80(6):1000-12. doi: 10.1124/mol.111.074708. Epub 2011 Aug 29.
|
|
|
|
|
|
|