| 1 |
ClinicalTrials.gov (NCT01072136) Empiric Therapy of Mucopurulent Cervicitis (MPC)
|
| 2 |
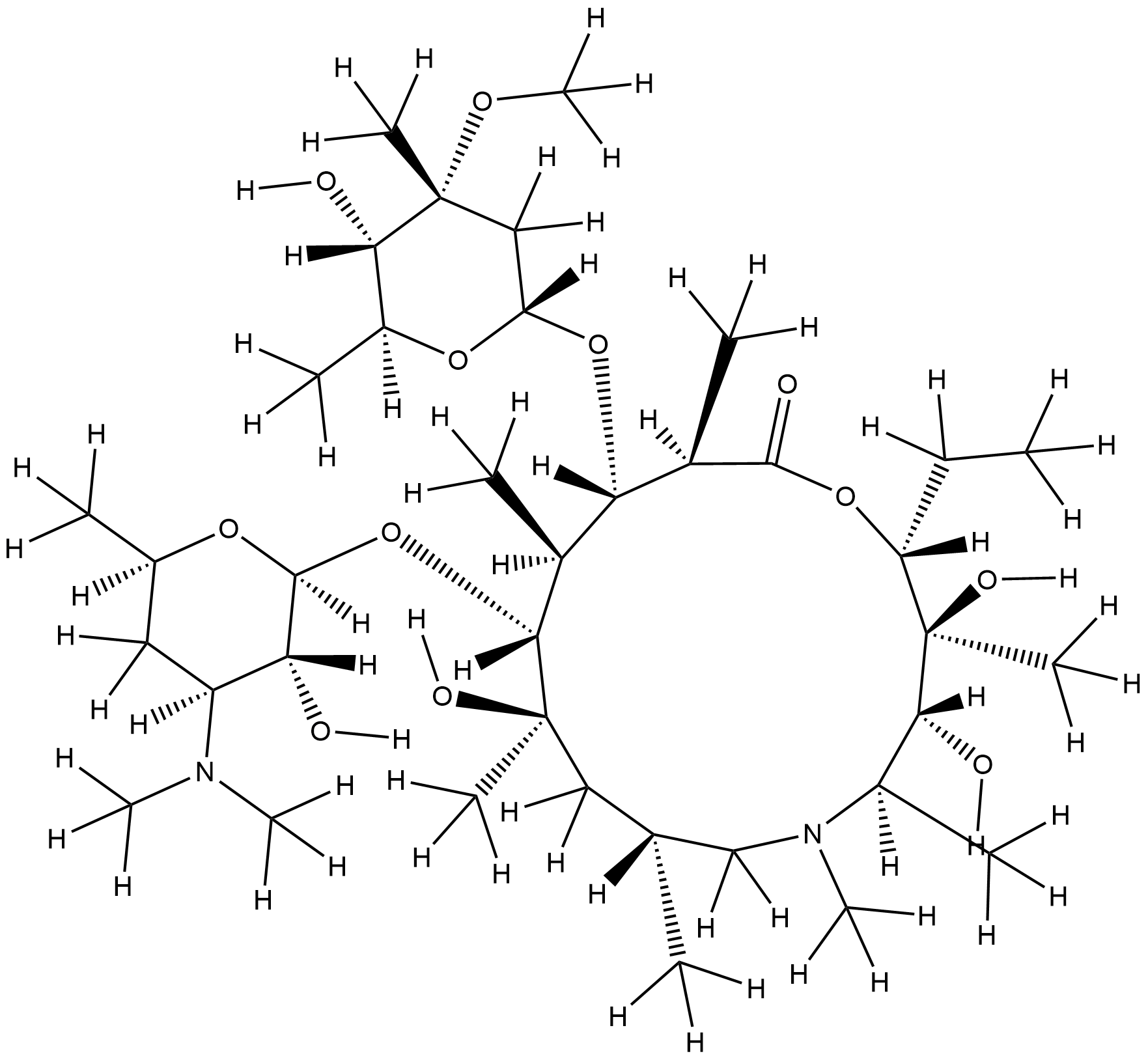
Azithromycin FDA Label
|
| 3 |
FDA Approved Drug Products from FDA Official Website. 2019. Application Number: (ANDA) 065511.
|
| 4 |
ClinicalTrials.gov (NCT04332107) Azithromycin for COVID-19 Treatment in Outpatients Nationwide. U.S. National Institutes of Health.
|
| 5 |
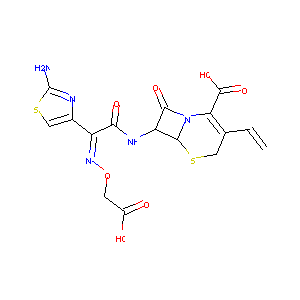
Cefixime FDA Label
|
| 6 |
Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77.
|
| 7 |
Genetics of chromosomally mediated intermediate resistance to ceftriaxone and cefixime in Neisseria gonorrhoeae. Antimicrob Agents Chemother. 2009 Sep;53(9):3744-51.
|
| 8 |
Flavonoids with epidermal growth factor-receptor tyrosine kinase inhibitory activity stimulate PEPT1-mediated cefixime uptake into human intestinal epithelial cells. J Pharmacol Exp Ther. 2001 Oct;299(1):351-7.
|
| 9 |
Transport characteristics of a novel peptide transporter 1 substrate, antihypotensive drug midodrine, and its amino acid derivatives. J Pharmacol Exp Ther. 2006 Jul;318(1):455-60.
|
|
|
|
|
|
|