| 1 |
ClinicalTrials.gov (NCT01242202) A Study to Assess the Safety and Efficacy of ASP1941 in Combination With -glucosidase Inhibitor in Type 2 Diabetic Patients
|
| 2 |
ClinicalTrials.gov (NCT01054092) A Study to Assess the Long-term Safety and Efficacy of ASP1941 in Japanese Diabetic Patients. U.S. National Institutes of Health.
|
| 3 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4842).
|
| 4 |
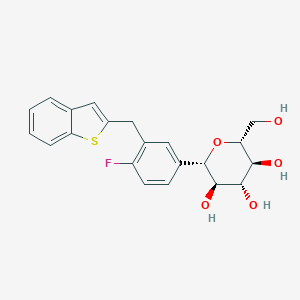
Ipragliflozin: A novel sodium-glucose cotransporter 2 inhibitor developed in Japan.World J Diabetes.2015 Feb 15;6(1):136-44.
|
| 5 |
Drug therapy of postprandial hyperglycaemia. Drugs. 1999 Jan;57(1):19-29.
|
| 6 |
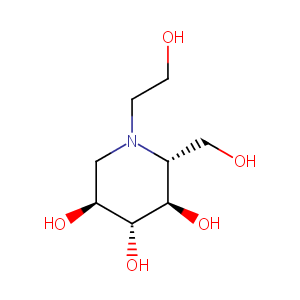
The -glucosidase inhibitor miglitol decreases glucose fluctuations and inflammatory cytokine gene expression in peripheral leukocytes of Japanese patients with type 2 diabetes mellitus. Metabolism. 2010 Dec;59(12):1816-22. doi: 10.1016/j.metabol.2010.06.006. Epub 2010 Jul 29.
|
|
|
|
|
|
|