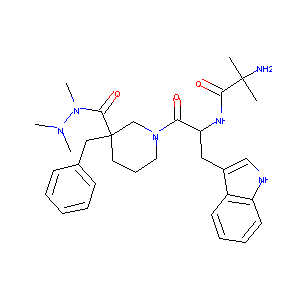
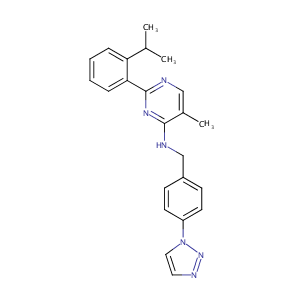
| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
ClinicalTrials.gov (NCT01395914) Anamorelin HCl in the Treatment of Non-Small Cell Lung Cancer-Cachexia (NSCLC-C): An Extension Study (ROMANA 3). U.S. National Institutes of Health.
|
| 3 |
Deubiquitylating enzymes and drug discovery: emerging opportunities. Nat Rev Drug Discov. 2018 Jan;17(1):57-78.
|
| 4 |
Pharmacodynamic hormonal effects of anamorelin, a novel oral ghrelin mimetic and growth hormone secretagogue in healthy volunteers. Growth Horm IGF Res. 2009 Jun;19(3):267-73.
|
| 5 |
Absorption, elimination, and metabolism of CS-1036, a novel -amylase inhibitor in rats and monkeys, and the relationship between gastrointestinal distribution and suppression of glucose absorption.Drug Metab Dispos.2013 Apr;41(4):878-87.
|
| 6 |
Synthesis and structure-activity relationship studies of N-benzyl-2-phenylpyrimidin-4-amine derivatives as potent USP1/UAF1 deubiquitinase inhibitors with anticancer activity against nonsmall cell lung cancer. J Med Chem. 2014 Oct 9;57(19):8099-110.
|
|
|
|
|
|
|