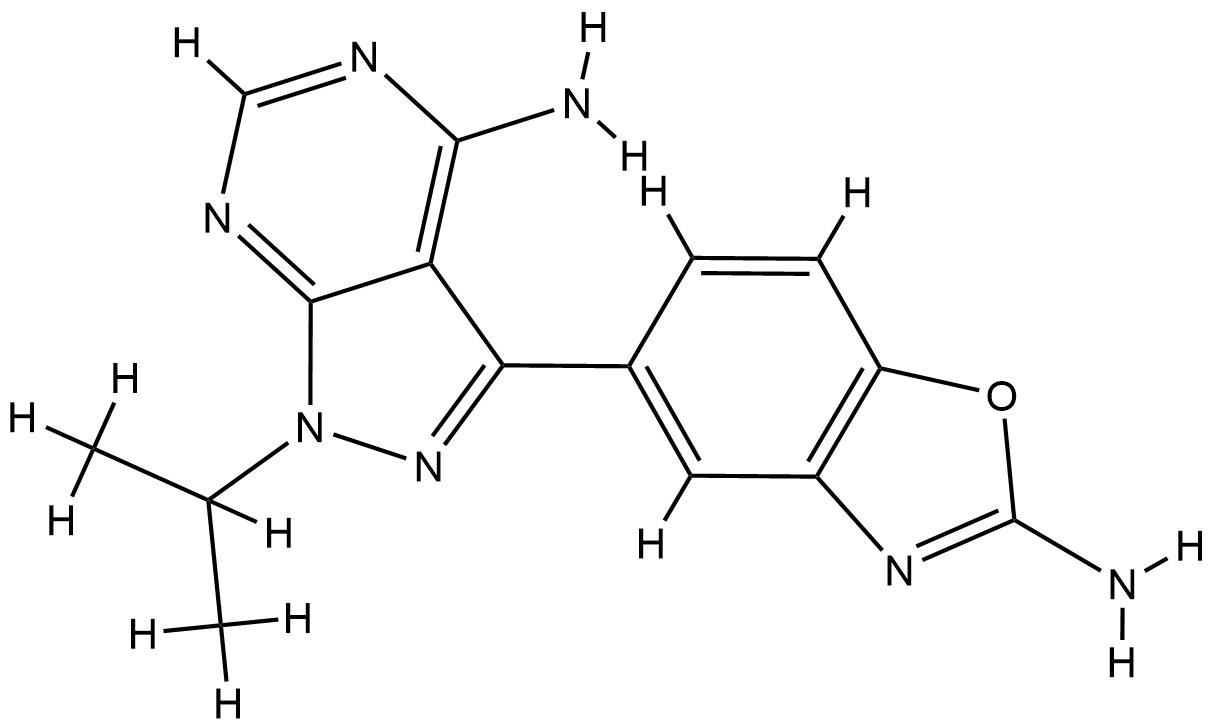
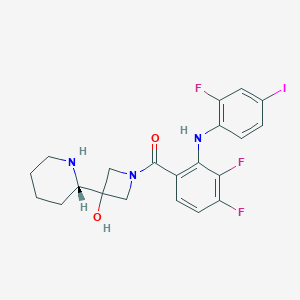
| 1 |
Loss of function mutations in VARS encoding cytoplasmic valyl-tRNA synthetase cause microcephaly, seizures, and progressive cerebral atrophy.Hum Genet. 2018 Apr;137(4):293-303. doi: 10.1007/s00439-018-1882-3. Epub 2018 Apr 24.
|
| 2 |
ClinicalTrials.gov (NCT02049957) Safety and Efficacy Study of Sapanisertib in Combination With Exemestane or Fulvestrant in Postmenopausal Women With Estrogen Receptor Positive/Human Epidermal Growth Factor Receptor 2 Negative (ER+/HER2-) Metastatic Breast Cancer. U.S. National Institutes of Health.
|
| 3 |
Prevent COVID-19 Severity by Repurposing mTOR Inhibitors. 2 April 2020
|
| 4 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 5 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 6 |
ClinicalTrials.gov (NCT01106599) A Study Evaluating the Safety, Tolerability, and Pharmacokinetics of GDC-0623 in Patients With Locally Advanced or Metastatic Solid Tumors. U.S. National Institutes of Health.
|
|
|
|
|
|
|