| 1 |
ClinicalTrials.gov (NCT05677282) Single Dose Antibiotic Treatment of Acute Watery Diarrhea, Rifaximin Versus Azithromycin, With Loperamide Adjunct
|
| 2 |
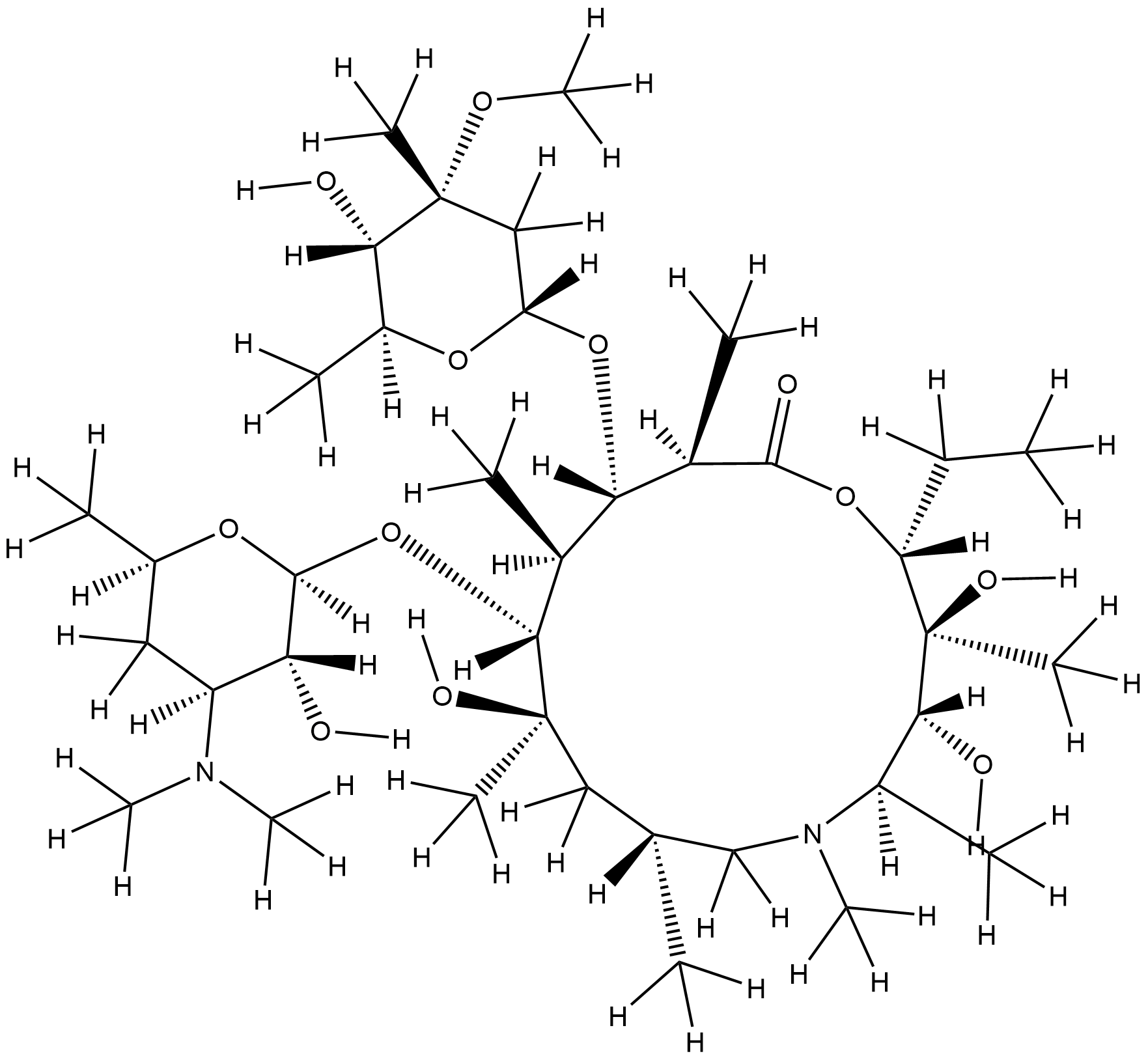
Azithromycin FDA Label
|
| 3 |
FDA Approved Drug Products from FDA Official Website. 2019. Application Number: (ANDA) 065511.
|
| 4 |
ClinicalTrials.gov (NCT04332107) Azithromycin for COVID-19 Treatment in Outpatients Nationwide. U.S. National Institutes of Health.
|
| 5 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 6 |
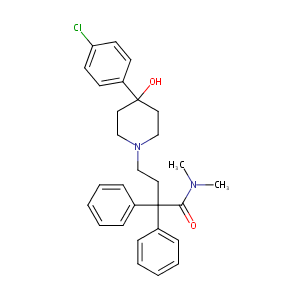
Loperamide FDA Label
|
| 7 |
Screening of an FDA-approved Compound Library Identifies Four Small-Molecule Inhibitors of Middle East Respiratory Syndrome Coronavirus Replication in Cell Culture Antimicrob Agents Chemother. 2014 Aug;58(8):4875-84.
|
| 8 |
Loperamide: evidence of interaction with mu and delta opioid receptors. Life Sci. 1983;33 Suppl 1:315-8.
|
| 9 |
In vitro P-glycoprotein assays to predict the in vivo interactions of P-glycoprotein with drugs in the central nervous system. Drug Metab Dispos. 2008 Feb;36(2):268-75.
|
| 10 |
Loperamide: a pharmacological review. Rev Gastroenterol Disord. 2007;7 Suppl 3:S11-8.
|
| 11 |
Identification of cytochrome P450 isoforms involved in the metabolism of loperamide in human liver microsomes. Eur J Clin Pharmacol. 2004 Oct;60(8):575-81.
|
| 12 |
Reduction of the prodrug loperamide oxide to its active drug loperamide in the gut of rats, dogs, and humans. Drug Metab Dispos. 1995 Mar;23(3):354-62.
|
|
|
|
|
|
|