| 1 |
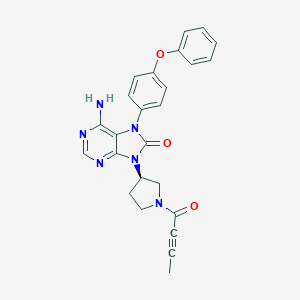
ClinicalTrials.gov (NCT02457598) Dose Escalation and Dose Expansion Study of Tirabrutinib in Combination With Other Targeted Anti-cancer Therapies in Adults With B-cell Malignancies
|
| 2 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 3 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 4 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7889).
|
| 5 |
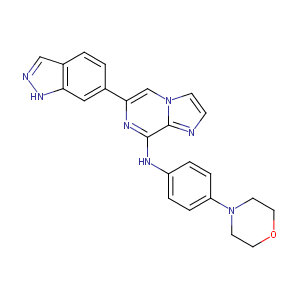
An open-label phase 2 trial of entospletinib (GS-9973), a selective spleen tyrosine kinase inhibitor, in chronic lymphocytic leukemia. Blood. 2015 Apr 9;125(15):2336-43.
|
|
|
|
|
|
|