| 1 |
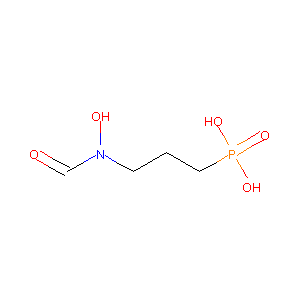
ClinicalTrials.gov (NCT01464125) Fosmidomycin and Azithromycin for Acute Uncomplicated Plasmodium Falciparum Malaria (P. Malaria) in Adults
|
| 2 |
The fight against drug-resistant malaria: novel plasmodial targets and antimalarial drugs. Curr Med Chem. 2008;15(2):161-71.
|
| 3 |
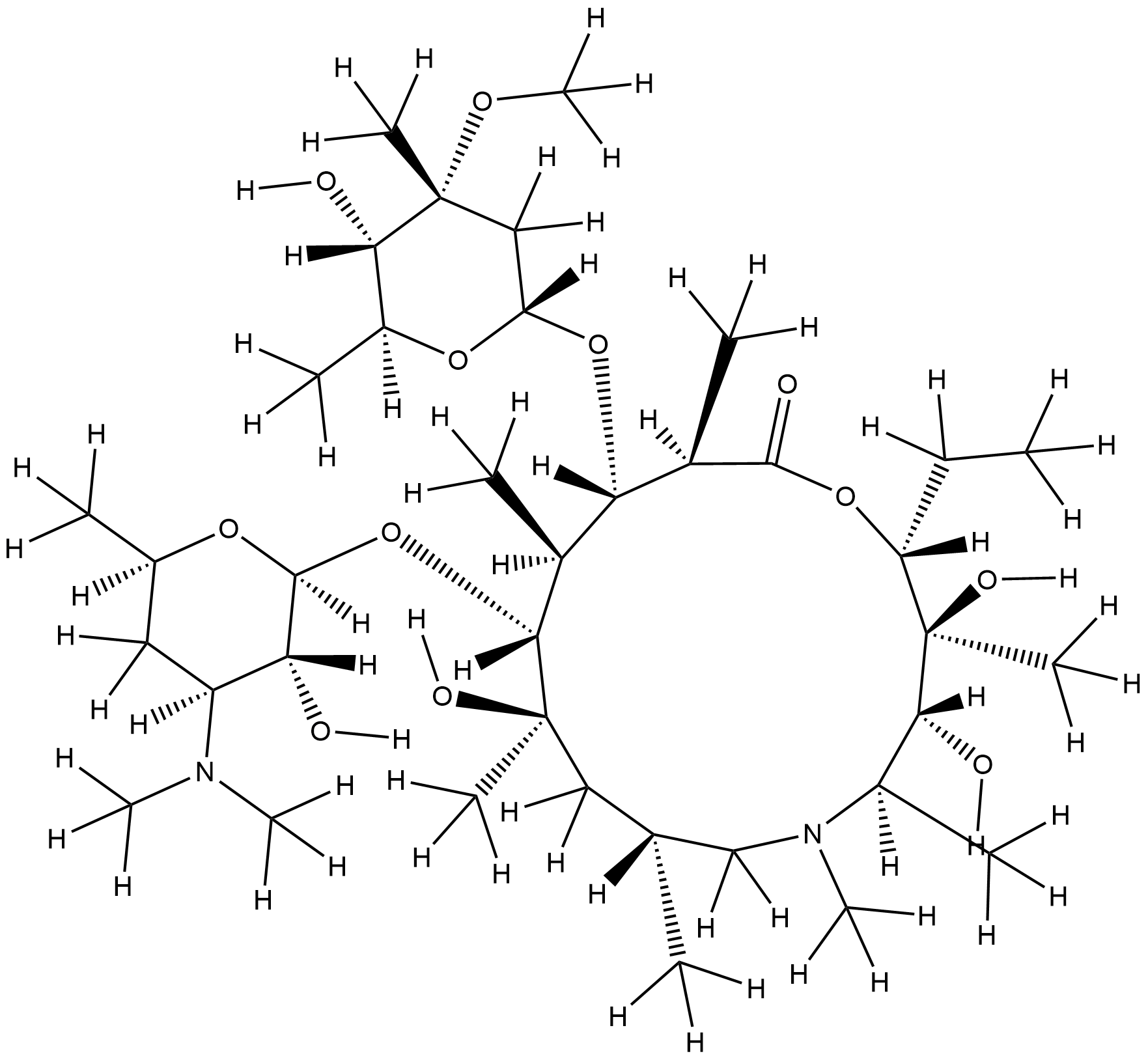
Azithromycin FDA Label
|
| 4 |
FDA Approved Drug Products from FDA Official Website. 2019. Application Number: (ANDA) 065511.
|
| 5 |
ClinicalTrials.gov (NCT04332107) Azithromycin for COVID-19 Treatment in Outpatients Nationwide. U.S. National Institutes of Health.
|
| 6 |
Structural basis of fosmidomycin action revealed by the complex with 2-C-methyl-D-erythritol 4-phosphate synthase (IspC). Implications for the cata... J Biol Chem. 2003 May 16;278(20):18401-7.
|
|
|
|
|
|
|