Details of the Drug Combinations
General Information of This Drug (ID: DM0HS92)
| Drug Name | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Merimebodib; Merimepodib [USAN:INN]; Tyverb/Tykerb; MMPD; 198821-22-6; 2ZL2BA06FU; C23H24N4O6; CHEMBL304087; MERIMEPODIB, VI-21497, VX-497; UNII-2ZL2BA06FU; VI-21497; VX-497; VX497; Vx 497; carbamic acid
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
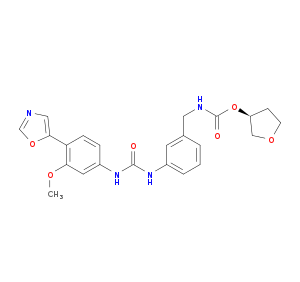 |
|||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
2 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||
References
