Details of the Drug Combinations
General Information of This Drug (ID: DM0LPI9)
| Drug Name | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
UNII-W6I5QDT7QI; W6I5QDT7QI; Netarsudil mesylate; Netarsudil [USAN]; AR-11324 free base; Netarsudil [USAN:INN]; Netarsudil (USAN/INN); GTPL9322; SCHEMBL16036278; ZINC113149554; AR11324; AR-11324; ester 60; 1628418-15-4
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Structure |
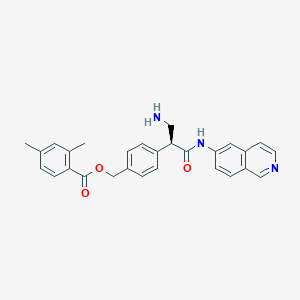 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
|||||||||||||||||||||||||
References
