Details of the Drug Combinations
General Information of This Drug (ID: DM1DBV5)
| Drug Name | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
dexlansoprazole; (R)-Lansoprazole; 138530-94-6; Kapidex; dexilant; R-(+)-LANSOPRAZOLE; Dexilant Solutab; TAK 390; UNII-UYE4T5I70X; TAK-390; (r)-(+)-lansoprazole; T 168390; UYE4T5I70X; AK170558; TAK-390MR; T-168390; 2-((R)-((3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridinyl)methyl)sulfinyl)-1H-benzimidazole; 2-[(R)-[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridyl]methylsulfinyl]-1H-benzimidazole; (R)-2-(((3-Methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl)methyl)sulfinyl)-1H-benzo[d]imidazole; Dexlansoprazole (INN/USAN); Kapidex; KS-1075; Lansoprazole
|
||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||
| Structure |
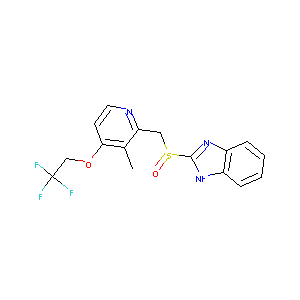 |
||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||
References
