Details of the Drug Combinations
General Information of This Drug (ID: DM2LU8M)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Rimiducid; AP1903; 195514-63-7; UNII-H564L1W5J2; AP 1903; AP-1903; H564L1W5J2; Rimiducid [INN]; Rimiducid [USAN:INN]; CHEMBL269259; SCHEMBL10111062; DTXSID80173226; EX-A1711; AKOS030238859; CS-3162; KB-74710; HY-16046; Z-3163; 2-Piperidinecarboxylic acid, 1-(1-oxo-2-(3,4,5-trimethoxyphenyl)butyl)-, 1,2-ethanediylbis(imino(2-oxo-2,1-ethanediyl)oxy-3,1-phenylene(3-(3,4-dimethoxyphenyl)propylidene)) ester, (2S-(1(R*),2R*(S*(S*(1(R*),2R*)))))-
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Structure |
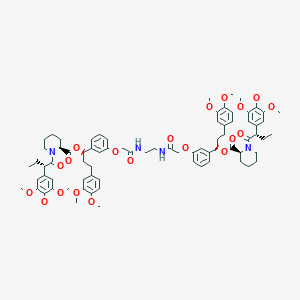 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
2 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||
References
