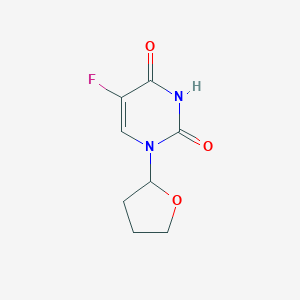
| 1 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 2 |
ClinicalTrials.gov (NCT03001726) To Observe the Efficacy and Safety by Comparing Chemotherapy(Docetaxel, Oxaliplatin Plus S1 ) Followed With Radical Resection Versus Chemotherapy Alone in Advanced Gastric Cancer With Single Non-curable Factor.
|
| 3 |
ClinicalTrials.gov (NCT00378716) Combination Chemotherapy in Treating Patients With Resected Colon Cancer
|
| 4 |
ClinicalTrials.gov (NCT03406299) Phase II Randomized Trial of SLOG vs GC in Locally Advanced or Metastatic Biliary Tract Cancer
|
| 5 |
ClinicalTrials.gov (NCT02623153) Comparison Between XELOX and S1, Oxaliplatin and Docetaxel as Neoadjuvant Chemotherapy for Gastric Cancer
|
| 6 |
ClinicalTrials.gov (NCT04216758) Nab-P and Gem Compared With Gem and Tegafur in Adjuvant Chemotherapy After Radical Resection of Pancreatic Cancer
|
| 7 |
ClinicalTrials.gov (NCT03204032) A Study of Tegafur Combined With Temozolomide Versus Tegafur Combined With Temozolomide and Thalidomide in Subjects With Advanced Extrapancreatic Neuroendocrine Tumor
|
| 8 |
ClinicalTrials.gov (NCT05914610) Envollizumab Combined With Fruquintinib and SOX Versus SOX for Conversion Therapy in Advanced Gastric Cancer
|
|
|
|
|
|
|