Details of the Drug Combinations
General Information of This Drug (ID: DM3WP0N)
| Drug Name | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Anlotinib; 1058156-90-3; UNII-GKF8S4C432; GKF8S4C432; AL-3818; SCHEMBL2063386; GTPL9601; KSMZEXLVHXZPEF-UHFFFAOYSA-N; MolPort-044-567-604; ZINC117924202; AKOS030526233; DB11885; CS-5396; AL 3818; HY-19716; 1-[[4-[(4-fluoro-2-methyl-1H-indol-5-yl)oxy]-6-methoxyquinolin-7-yl]oxymethyl]cyclopropan-1-amine; 1-((4-(4-fluoro-2-methyl-1h-indol-5-yloxy)-6-methoxyquinolin-7-yloxy)methyl)cyclopropan-amine; Cyclopropanamine, 1-(((4-((4-fluoro-2-methyl-1H-indol-5-yl)oxy)-6-methoxy-7-quinolinyl)oxy)methyl)-
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
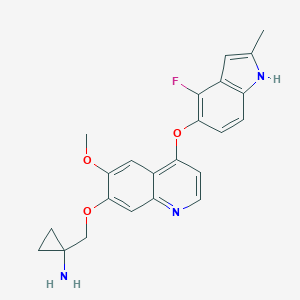 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
17 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
