Details of the Drug Combinations
General Information of This Drug (ID: DM3XRJT)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Pravadoline; 92623-83-1; (4-Methoxyphenyl)(2-methyl-1-(2-morpholinoethyl)-1H-indol-3-yl)methanone; UNII-P3JW662TWA; WIN 48098; P3JW662TWA; CHEMBL13178; NCGC00160396-01; DSSTox_RID_81361; DSSTox_CID_26127; DSSTox_GSID_46127; Pravadoline, Pravadoline Maleate; Pravadolina; Pravadolinum; (4-methoxyphenyl)-[2-methyl-1-(2-morpholin-4-ylethyl)indol-3-yl]methanone; SMR001550504; Pravadoline [INN]; CAS-92623-83-1; Pravadolinum [INN-Latin]; Pravadolina [INN-Spanish]; AC1Q5EGD; MLS006010335; MLS004774040; SCHEMBL488940; Pravadoline(WIN 48 09
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
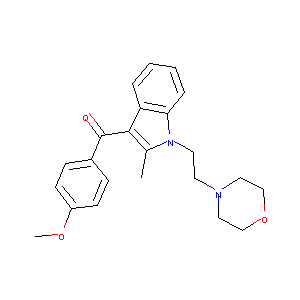 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
2 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
||||||||||||||||||||||||||||||
References
