Details of the Drug Combinations
General Information of This Drug (ID: DM4HYMS)
| Drug Name | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Veliparib; 912444-00-9; ABT-888; ABT 888; ABT-888 (Veliparib); Veliparib (ABT-888); ABT888; UNII-01O4K0631N; 2-[(2R)-2-Methylpyrrolidin-2-yl]-1H-benzimidazole-4-carboxamide; CHEBI:62880; 2-[(R)-2-methylpyrrolidin-2-yl]-1H-benzimidazole-4-carboxamide; (R)-2-(2-methylpyrrolidin-2-yl)-1H-benzo[d]imidazole-4-carboxamide; 01O4K0631N; 2-[(2R)-2-Methylpyrrolidin-2-yl]-1H-benimidazole-4-; (2r)-2-(7-Carbamoyl-1h-Benzimidazol-2-Yl)-2-Methylpyrrolidinium; Veliparib dihydrochloride
|
||||||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||||||
| Structure |
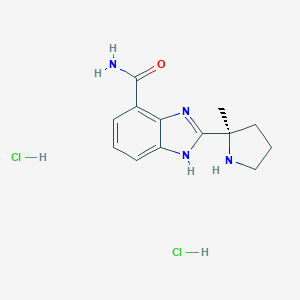 |
||||||||||||||||||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
11 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
