Details of the Drug Combinations
General Information of This Drug (ID: DM4W9B3)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Telzir; Amprenavir phosphate; Fosamprenavir [INN]; GW 433908; GW433908; VX 175; Fosamprenavir (INN); Lexiva (TM); Lexiva (TN); Telzir (TN); Telzir(TM); VX-175; GW433908A (*Sodium Salt*); GW433908G (*Calcium Salt*); [(3S)-oxolan-3-yl] N-[(2S,3R)-4-[(4-aminophenyl)sulfonyl-(2-methylpropyl)amino]-1-phenyl-3-phosphonooxybutan-2-yl]carbamate; Carbamic acid, ((1S,2R)-3-(((4-aminophenyl)sulfonyl)(2-methylpropyl)amino)-1-(phenylmethyl)-2-(phosphonooxy)propyl)-, C-((3S)-tetrahydro-3-furanyl) ester; ((3S)Oxolan-3-yloxy)-N-((1S,2R)-3-{[(4-aminophenyl)sulfonyl](2-methylpropyl)amino}-1-benzyl-2-(phosphonooxy)propyl)carboxamide; (3-(((4-Aminophenyl)sulfonyl)(2-methylpropyl)amino)-1-(phenylmethyl)-2-(phosphonooxy)propyl)carbamic acid C-(tetrahydro-3-furanyl) ester
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Anti-HIV Agents
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
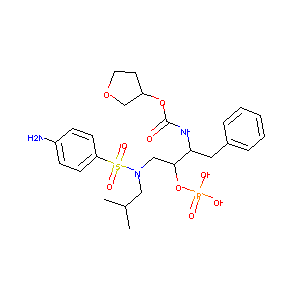 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||
