Details of the Drug Combinations
General Information of This Drug (ID: DM5H0R9)
| Drug Name | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
LY317615; LE-0014; LY317615, Enzastaurin; 3-(1-methyl-1H-indol-3-yl)-4-{1-[1-(pyridin-2-ylmethyl)piperidin-4-yl]-1H-indol-3-yl}-1H-pyrrole-2,5-dione; 3-(1-methylindol-3-yl)-4-[1-[1-(pyridin-2-ylmethyl)piperidin-4-yl]indol-3-yl]pyrrole-2,5-dione
|
||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Anticancer Agents
|
||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||
| Structure |
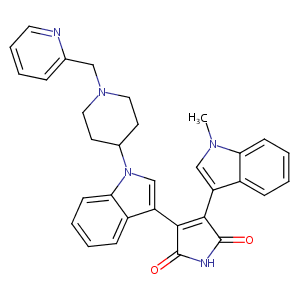 |
||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
9 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
