Details of the Drug Combinations
General Information of This Drug (ID: DM5NW1E)
| Drug Name | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
BECLOMETHASONE DIPROPIONATE; Beclometasone dipropionate; Beconase; Beclovent; Vancenase; Beclorhinol; Becloforte; Sanasthmyl; Beclazone; Sanasthmax; Propaderm; Beclomet; Vanceril; Aerobec; Becloturmant; Beclacin; Respocort; Entyderma; Korbutone; Viarox; Rino-clenil; Clenil-A; Beclometasone 17,21-dipropionate; Becotide; Aldecin; Turbinal; Aldecina; Rhinosol; Beclate; Atomase; Alanase; Benconase; Aldecine; Clenil; Spir; Beclocort Nasel; Beclomet Nasal; Propaderm Forte; Rhino Clenil; Inalone O; Inalone R; Qvar; QNASL; Beconase AQ; Qvar 80
|
||||||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||||||
| Structure |
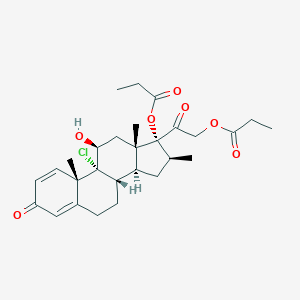 |
||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
3 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||
References
