Details of the Drug Combinations
General Information of This Drug (ID: DM6AVR4)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
hydroquinone; 1,4-benzenediol; Benzene-1,4-diol; 123-31-9; 1,4-Dihydroxybenzene; Quinol; p-Benzenediol; p-Hydroxyphenol; p-Hydroquinone; 4-Hydroxyphenol; Eldoquin; Benzoquinol; p-Dihydroxybenzene; hydroquinol; Eldopaque; Phiaquin; p-Dioxybenzene; Hydroquinole; Dihydroquinone; Tecquinol; Idrochinone; Benzohydroquinone; Tequinol; Hidroquinone; Arctuvin; Dihydroxybenzene; Solaquin forte; Derma-Blanch; Tenox HQ; Hydrochinone; Hydrochinon; Artra; Eldoquin Forte; Eldopaque Forte; Diak 5; Benzene, p-dihydroxy-; 1,4-Dihydroxy-benzol; Usaf ek-356; Accutin
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
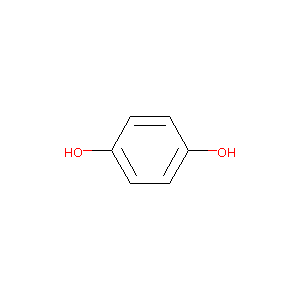 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||
