Details of the Drug Combinations
General Information of This Drug (ID: DM6LDI4)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
KGERZPVQIRYWRK-GDLZYMKVSA-N; 1800340-40-2; UNII-X8BW0MQ5PI; X8BW0MQ5PI; SCHEMBL16861831; EX-A2678; CS-7497; HY-101567; (S)-2-(3-(1,4-dimethyl-1H-1,2,3-triazol-5-yl)-5-(phenyl(tetrahydro-2H-pyran-4-yl)methyl)-5H-pyrido[3,2-b]indol-7-yl)propan-2-ol
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Structure |
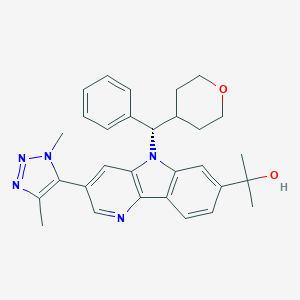 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
2 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||
References
