Details of the Drug Combinations
General Information of This Drug (ID: DM6OFJ8)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Indibulin; 204205-90-3; 2-(1-(4-chlorobenzyl)-1H-indol-3-yl)-2-oxo-N-(pyridin-4-yl)acetamide; d-24851; UNII-80K4H2RB8P; 80K4H2RB8P; ZIO-301; D 24851; 2-[1-(4-chlorobenzyl)-1h-indol-3-yl]-2-oxo-n-(pyridin-4-yl)acetamide; N-(Pyridin-4-yl)-[1-(4-chlorobenzyl)-indol-3-yl]-glyoxyl Amide; Indibulin [USAN:INN]; 2-{1-[(4-chlorophenyl)methyl]indol-3-yl}-2-oxo-N-(4-pyridyl)acetamide; 2-[1-[(4-chlorophenyl)methyl]indol-3-yl]-2-oxo-N-(4-pyridyl)acetamide; Indibulin (USAN/INN); AC1L1ESK; AC1Q5NS8; MLS006011745; CHEMBL49642
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
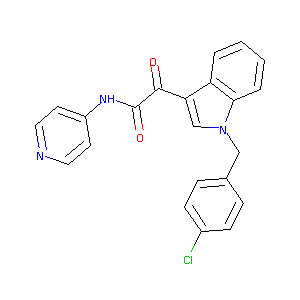 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
2 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||
