Details of the Drug Combinations
General Information of This Drug (ID: DM6YI5T)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Tegobuvir; 1000787-75-6; UNII-5NOK5X389M; 5-((6-(2,4-bis(Trifluoromethyl)phenyl)pyridazin-3-yl)methyl)-2-(2-fluorophenyl)-5H-imidazo[4,5-c]pyridine; 5NOK5X389M; GS-9190; GS-333126; W-204340; 5H-Imidazo[4,5-c]pyridine, 5-[[6-[2,4-bis(trifluoromethyl)phenyl]-3-pyridazinyl]methyl]-2-(2-fluorophenyl)-;5H-Imidazo[4,5-c]pyridine, 5-[[6-[2,4-bis(trifluoromethyl)phenyl]-3-pyridazinyl]methyl]-2-(2-fluorophenyl)-; Tegobuvir [USAN:INN]; GS 9190; Tegobuvir (USAN/INN); GS 333126; SCHEMBL496778; CHEMBL1957287; DTXSID80142917
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
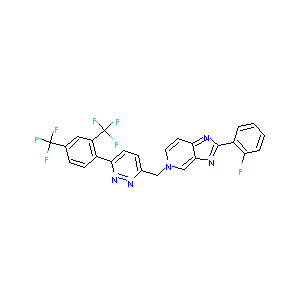 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
|||||||||||||||||||||||||
