Details of the Drug Combinations
General Information of This Drug (ID: DM726HX)
| Drug Name | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Mocetinostat; MG 0103; MG 4230; MG 4915; MG 5026; MG0103; MG4230; MG4915; MG5206; MGCD 0103; MGCD0103; MG-0103; MG-4230; MG-4915; MG-5026; Mocetinostat, MGCD0103; N-(2-aminophenyl)-4-[[(4-pyridin-3-ylpyrimidin-2-yl)amino]methyl]benzamide; N-(2-Aminophenyl)-4-((4-pyridin-3-ylpyrimidin-2-ylamino)methyl)benzamide
|
||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Anticancer Agents
|
||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||
| Structure |
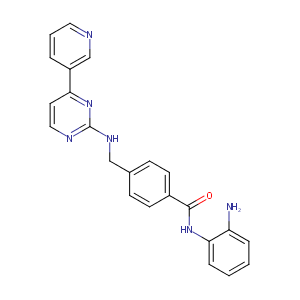 |
||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
2 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||
References
