Details of the Drug Combinations
General Information of This Drug (ID: DM73UTG)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Pralnacasan; VX-740; UNII-N986NI319S; 192755-52-5; N986NI319S; HMR3480/VX-740; Pralnacasan [USAN:INN]; HMR 3480; VX 470; Pralnacasan (USAN/INN); AC1L4A1A; SCHEMBL142187; GTPL6467; CHEMBL437526; DTXSID60172873; HMR3480; HMR-3480; BDBM50189360; AKOS030230853; DB04875; D08978; (4S,7S)-N-[(2R,3S)-2-ethoxy-5-oxooxolan-3-yl]-7-(isoquinoline-1-carbonylamino)-6,10-dioxo-2,3,4,7,8,9-hexahydro-1H-pyridazino[1,2-a]diazepine-4-carboxamide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
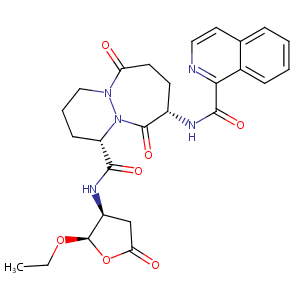 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
|||||||||||||||||||||||||
References
