Details of the Drug Combinations
General Information of This Drug (ID: DM7I1T9)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
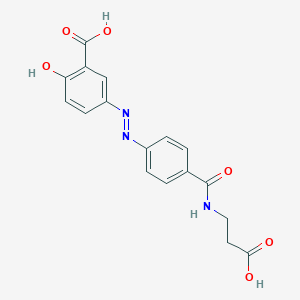
Balsalazida; Balsalazida [Spanish]; Balsalazide Disodium; Balsalazide [INN:BAN]; Balsalazido; Balsalazido [Spanish]; Balsalazidum; Balsalazidum [Latin]; BX-661A; Colazide; BALSALAZIDE; P80AL8J7ZP; (E)-5-((4-(((2-Carboxyethyl)amino)carbonyl)phenyl)azo)-2-hydroxybenzoic acid; (E)-5-((4-((2-Carboxyethyl)carbamoyl)phenyl)diazenyl)-2-hydroxybenzoic acid; (E)-5-({p-[(2-carboxyethyl)carbamoyl]phenyl}azo)-2-salicylic acid; 80573-04-2; C17H15N3O6; CHEBI:267413; UNII-P80AL8J7ZP
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Structure |
 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
|||||||||||||||||||||||||
