Details of the Drug Combinations
General Information of This Drug (ID: DM96JTX)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Atevirdine; 136816-75-6; Atevirdine mesylate; UNII-N24015WC6D; CHEMBL280527; N24015WC6D; U-87201E; 1-[(5-Methoxyindol-2-yl)carbonyl]-4-[3-(ethylamino)-2-pyridyl]piperazine; [4-[3-(ethylamino)-2-pyridyl]piperazin-1-yl]-(5-methoxy-1H-indol-2-yl)methanone; Atevirdine [INN]; Piperazine, 1-(3-(ethylamino)-2-pyridinyl)-4-((5-methoxy-1H-indol-2-yl)carbonyl)-; Piperazine, 1-[3-(ethylamino)-2-pyridinyl]-4-[(5-methoxy-1H-indol-2-yl)carbonyl]-; AC1Q5KK9; AC1L1U1S; SCHEMBL356038; bis(heteroaryl)piperazine analog; BDBM1437
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
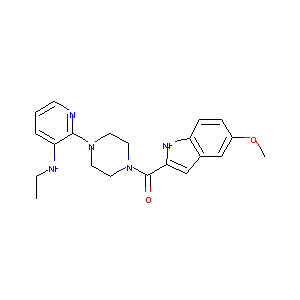 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||
References
