Details of the Drug Combinations
General Information of This Drug (ID: DM9AJRB)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Zinc sulfate; Zinc sulphate; 7733-02-0; Zincate; White vitriol; Zinc sulfate (1:1); Complexonat; Zinklet; Orazinc; Bonazen; Zinkosite; Zincomed; Solvezinc; Solvezink; Optraex; Optised; Medizinc; Neozin; ZnSO4; Zinc sulfate anhydrous; White copperas; Zinc vitriol; Zinci Sulfas; Visine-ac; Prefrin-Z; Zincum Sulfuricum; Zink-Gro; OP-Thal-zin; Bufopto zinc sulfate; Sulfuric acid, zinc salt (1:1); Zinc vitriol (VAN); component of Phenylzin; Caswell No. 927; Zinc Sulfate [USP]; White vitriol (VAN); Zinc sulfate (ZnSO4); Zinc-200; NU-Z
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
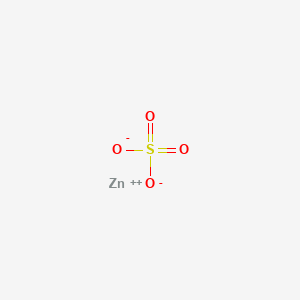 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
3 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||
References
