Details of the Drug Combinations
General Information of This Drug (ID: DMA12HL)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Azelnidipine; Azelnidipine [INN]; Calblock; RS-9054; 123524-52-7; 3,5-PYRIDINEDICARBOXYLIC ACID, 2-AMINO-1,4-DIHYDRO-6-METHYL-4-(3-NITROPHENYL)-, 3-[1-(DIPHENYLMETHYL)-3-AZETIDINYL] 5-(1-METHYLETHYL) ESTER; 3-(1-Benzhydrylazetidin-3-yl) 5-isopropyl 2-amino-6-methyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate; C33H34N4O6; CS 905; CS-905; DSSTox_CID_120; DSSTox_GSID_20120; DSSTox_RID_75382; MFCD00865803; NCGC00167436-01; NCGC00167436-02
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Structure |
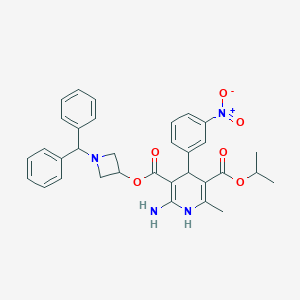 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
21 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
1 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
