Details of the Drug Combinations
General Information of This Drug (ID: DMA2Z4F)
| Drug Name | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Alopexil; Alostil; Loniten; Lonolox; Minodyl; Minossidile; Minoxidilum; Minoxigaine; Minoximen; Neoxidil; Normoxidil; Pierminox; Prexidil; Regaine; RiUP; Rogaine; Theroxidil; Tricoxidil; Trocoxidil; MINOXIDIL EXTRA STRENGTH FOR MEN; Men s Rogaine Foam; Mens Rogaine Foam; Minossidile [Italian]; Minoxidil Pfizer Brand; PfizerBrand of Minoxidil; Rogaine Extra Strength for Men; Rogaine for Men; Rogaine for Women; M 4145; M1389; U 10858; Apo-Gain; Gen-Minoxidil; Loniten (TN); MINOXIDIL (FOR MEN); MINOXIDIL (FOR WOMEN); MINOXIDIL EXTRA STRENGTH (FOR MEN); Men's Rogaine; Minoxidilum [INN-Latin]; ROGAINE (FOR MEN); ROGAINE (FOR WOMEN); ROGAINE EXTRA STRENGTH (FOR MEN); Regaine (TN); Riup (TN); Rogaine (TN); TM-160; U-10858; Minoxidil (USP/INN); Minoxidil [USAN:BAN:INN]; U-10,858; Rogaine, Regaine, Avacor and Mintop, Minoxidil; Pyrimidine, 2,4-diamino-6-piperidino-, 3-oxide; 2,3-Dihydro-3-hydroxy-2-imino-6-(1-piperidinyl)-4-pyrimidinamine; 2,4-Diamino-6-piperidinilpirimidina-3-ossido; 2,4-Diamino-6-piperidinilpirimidina-3-ossido [Italian]; 2,4-Diamino-6-piperidino-pyrimidine-3-oxide; 2,4-Diamino-6-piperidinopyrimidine 3-N-oxide; 2,4-Diamino-6-piperidinopyrimidine 3-oxide; 2,4-Pyrimidinediamine, 6-(1-piperidinyl)-, 3-oxide; 2,6-Diamino-4-piperidinopyrimidin-1-oxid; 3-hydroxy-2-imino-6-(1-piperidyl)pyrimidin-4-amine; 3-hydroxy-2-imino-6-piperidin-1-ylpyrimidin-4-amine; 6-(1-Piperidinyl)-2,4-pyrimidinediamine 3-oxide; 6-(piperidin-1-yl)pyrimidine-2,4-diamine 3-oxide; 6-Amino-1,2-dihydro-1-hydroxy-2-imino-4-piperidinopyrimidine; 6-Piperidino-2,4-diaminopyrimidine 3-oxide; 6-[1-Piperidinyl]pyrimidine-2,4-diamine 3 oxide
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Therapeutic Class |
Antihypertensive Agents
|
||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
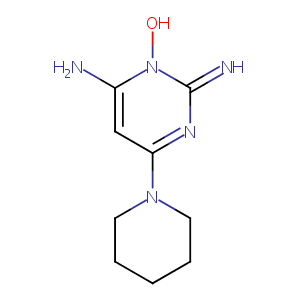 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
3 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||
References
